Ventricular assist device
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Marv Slepian, M.D.; University of Arizona [2]; Juan A. Sanchez MD MPA [3], Chairman, The Stanley J. Dudrick Department of Surgery, Saint Mary's Hospital, Waterbury, CT, Syed Hassan A. Kazmi BSc, MD [4]
Overview
A Ventricular assist device, or VAD, is a mechanical device that is used in cases of advanced heart failure. VADs are employed as a strategy of bridge to transplant or as a destination therapy in patients ineligible for transplant. It has been shown that about a quarter of patients waiting for heart transplants need mechanical circulatory support with left ventricular assist devices (LVAD) as bridge to transplant. Some VADs are designed for short term use, typically for patients recovering from heart attacks or heart surgery, while others are intended for long term use (months to years and in some cases for life), typically for patients suffering from congestive heart failure. Over the recent years, VADs have undergone significant improvements in size, durability, reliability and noise emission.
Clinical practice guidelines suggest roles[1].
VADs can be:
- Percutaneous or transcutaneous. Examples include[1]
- Axial flow pumps, such as Impella
- Left atrial to femoral artery bypass pumps, such as the TandemHeart
- Implantable that allows a patient to be fully mobile. Examples include:
- HeartMate II which is a continuous-flow system with mechanical bearings
- HeartMate III which is a magnetically levitated device that is a pulsatile-flow system without mechanical bearings
Design
- Most VADS operate on similar principles. A cannula is inserted into the apex of the appropriate ventricle. Blood passes through this to a pump and thence through a tube to the aorta (ascending aorta - most common) in the case of an LVAD or to the pulmonary artery in the case of an RVAD.
- The pump is powered through a lead which connects it to a controller and power supply. In some cases there is also a tube to vent the pump to the outside air.
- The Jarvik 2000 operates slightly differently - the pump is actually located inside the left ventricle, its outflow passes through the apex of the ventricle to a tube which leads to the aorta. Major distinguishing features between the different VADS are the pump (which can vary substantially in method of operation, size and placement), the controller, the materials used both for the pump and the associated tubes and cannulas and the lead between the pump and the controller/power supply.[2]
Classification
Classification based on pumps
Pumps used in VADS can be divided into two main categories - pulsatile pumps, which as the name suggests mimic the natural pulsing action of the heart, and continuous flow pumps[3][4].
Classification based on generation
First-generation devices
- First-generation devices are pulsatile volume displacement pumps that use positive displacement pumps. In some of these pumps the volume occupied by blood varies during the pumping cycle, and if the pump is contained inside the body (a median steronotomy is required) is then a vent tube to the outside air is required. The pumping chamber is located within the abdomen or preperitoneal space, and a single transcutaneous drive line exits the abdominal wall. Examples are Berlin Heart EXCOR, Novacor, LionHeart, HeartMate I
Second-generation devices
- Second-generation devices are axial, continuous flow pumps. Continuous flow VADs normally use either centrifugal pumps or axial flow pump. Both types have a central rotor containing permanent magnets. Controlled electric currents running through coils contained in the pump housing apply forces to the magnets which cause the rotors to rotate. In the centrifugal pumps the rotors are shaped to accelerate the blood circumferentially and thus cause it to move towards the outer rim of the pump whereas in the axial flow pumps the rotors are more or less cylindrical with blades that are more or less helical, causing the blood to be accelerated in the direction of the rotor's axis. Examples include MicroMed DeBakey, HeartMate II, Jarvik 2000, TandemHeart[5][6]
Third-generation devices
- Third-generation devices are bearingless pumps. An important issue with continuous flow pumps is the method used to suspend the rotor. Early versions used solid bearings however newer pumps, some of which are approved for use in the EU use either electromagnetic or hydrodynamic suspension. Manufacturers claim that these methods of suspension not only virtually eliminate wear but also reduce damage to blood cells. Third-generation devices are bearingless pumps. Examples include HeartWare HVAD® (HeartWare International, Inc., Framingham MA), Incor® (Berlin Heart, Inc., Berlin, Germany), Levacor® (World Heart Inc., Salt Lake City, UT), CentriMag, DuraHeart, HeartMate III, Impella[7]
Biventricular Assist Device (BiVAD)
Indications
VADs can be used for the following conditions:[8][9][10][11]
- Bridge-to-Transplantation
- Destination therapy (Heart failure with reduced ejection fraction)
- Bridge-to-the-Decision
- Bridge-to-Recovery
Contraindications
The following are the contraindications to VADs:[12]
- Acute cardiogenic shock with a neurological compromise
- Coexisting severe terminal comorbidity
- Bleeding and thrombocytopenia
- Hypertrophic cardiomyopathy or a large ventricular septal defect
- Body surface area less than 1.2 to 1.5 m2
- Right ventricular dysfunction
Effectiveness
Clinical practice guidelines summarize indications[13].
Systematic reviews have assessed effectiveness[3][14] including comparison of effectiveness to intra-aortic balloon pump[15].
Among studies of artificial hearts, most original studies are not randomized; however, one randomized controlled trial found a trend, although insignificant, for benefit from the Impella ventricular assist device compare to intra-aortic balloon pumping[16].
Complications
- Right Ventricular(RV) failure:
Unloading of the left ventricle post-LVAD implantation may result in septal shifts, leading to increased RV preload, decreased RV contractility and function. RVAD implantation may be necessary until RV function improves, at which point RVAD can be explanted. [17]
- Gastro-intestinal (GI) Bleed:
Patients with Continuous Flow LVADs are systemically anticoagulated with Warfarin, which increases the risk of GI Bleed. Patients should be evaluated and if possible, treated preoperatively for GI bleeding sources such as colonic polyps, stomach ulcers and angiodysplasias. There is also a higher risk of bleeding post-LVAD implantation from degradation of Von Willebrand Factor by VAD rotors, which is reversible once LVAD is removed.[17]
- Thromboemolism and Stroke:
There is an increased risk of thromboembolic events and Transient Ischemic Attacks in patients with Continous Flow LVADs despite of systemic anticoagulation. If pump thrombosis develops, there might be an emergent need for pump exchange and a relatively quicker heart transplant. [17]
- Infection
- Ventricular arrythmias
- Hypertension
Because the devices generally result in blood flowing over a non biologic surface, predisposing the blood to clotting, there is need for anticoagulation. There is one device, the Heartmate, which provides a biologic surface derived from fibrin and does not require long term anticoagulation; unfortunately, this biologic surface may predispose to infection.
VAD-related infection can be caused by a large number of different organisms:[18]
- Gram positive bacteria (Staphylococci especially Staph. aureus, Enterococci)
- Gram negative bacteria (Pseudomonas aeruginosa, Enterobacter species, Klebsiella species)
- Fungi especially Candida sp.
Treatment of VAD-related infection is exceedingly difficult and many patients die of infection despite optimal treatment. Initial treatment should be with broad spectrum antibiotics, but every effort must be made to obtain appropriate samples for culture. A final decision regarding antibiotic therapy must be based on the results of microbiogical cultures.
Other problems include immunosuppression, clotting with resultant stroke, and bleeding secondary to anticoagulation. It is interesting to note that some of the polyurethane components used in the devices cause the deletion of a subset of immune cells when blood comes in contact with them. This predisposes the patient to fungal and some viral infections necessitating appropriate prophylactic therapy.
History
The early VADs emulated the heart by using a "pulsatile" action where blood is alternately sucked into the pump from the left ventricle then forced out into the aorta. Devices of this kind include the Heartmate, which was approved for use in the US by the FDA in October 1994. These devices are commonly referred to as first generation VADs
More recent work has concentrated on continuous flow pumps, which can be roughly categorized as either centrifugal pumps or axial flow impeller driven pumps. These pumps have the advantage of greater simplicity resulting in smaller size and greater reliability. These devices are referred to as second generation VADs. A side effect is that their users need to carry documentation saying that the lack of a pulse does not mean that they are dead.
Third generation VADs suspend the impeller in the pump using either hydrodynamic or electromagnetic suspension, thus removing the need for bearings and reducing the number of moving parts to one.
Another technology undergoing clinical trials is the use of trans cutaneous induction to power and control the device rather than using percutaneous cables. Apart from the obvious cosmetic advantage this reduces the risk of infection and the consequent need to take preventative action. A pulsatile pump using this technology has CE Mark approval and is in clinical trials for US FDA approval.
A very different approach in the early stages of development is the use of an inflatable cuff around the aorta. Inflating the cuff contracts the aorta and deflating the cuff allows the aorta to expand - in effect the aorta becomes a second left ventricle. A proposed refinement is to use the patient's skeletal muscle, driven by a pacemaker, to power this device which would make it truly self contained. In any case it has substantial potential advantages in avoiding the need to operate on the heart itself and in avoiding any contact between blood and the device. Interestingly this approach involves a return to a pulsatile flow.
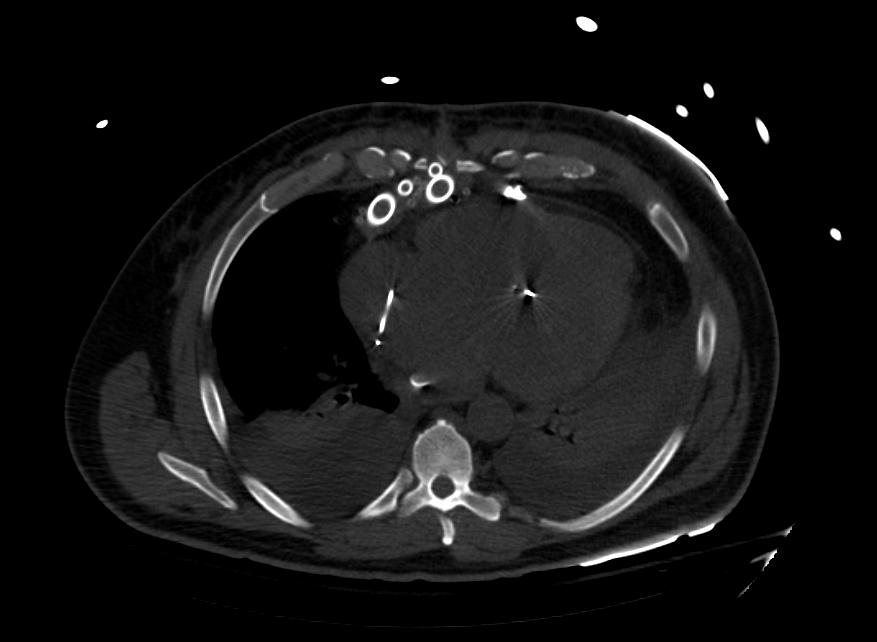
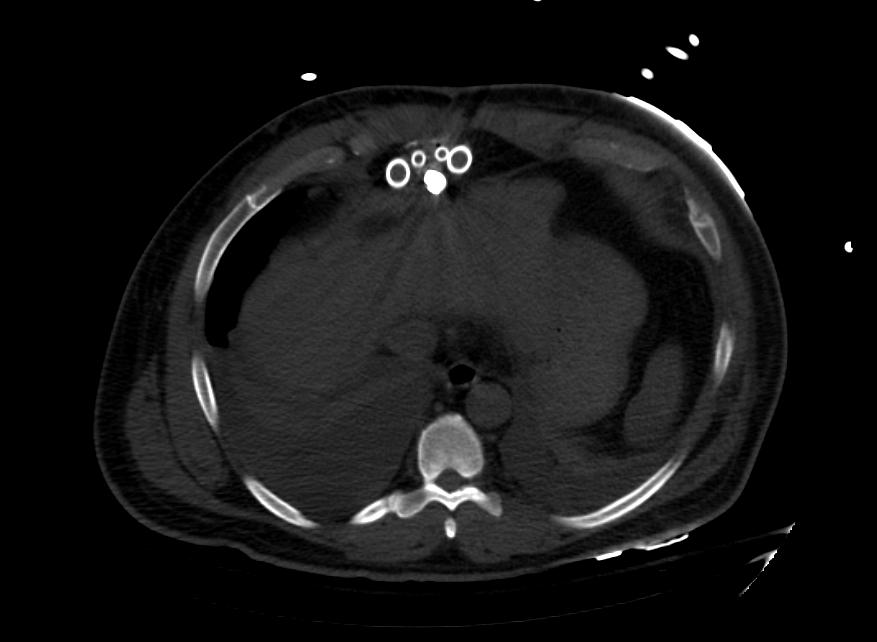
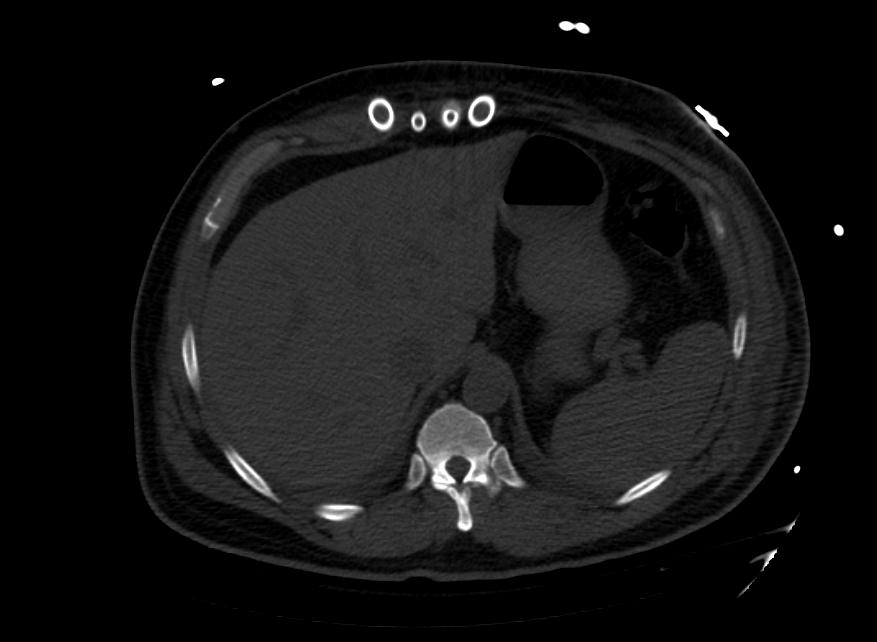
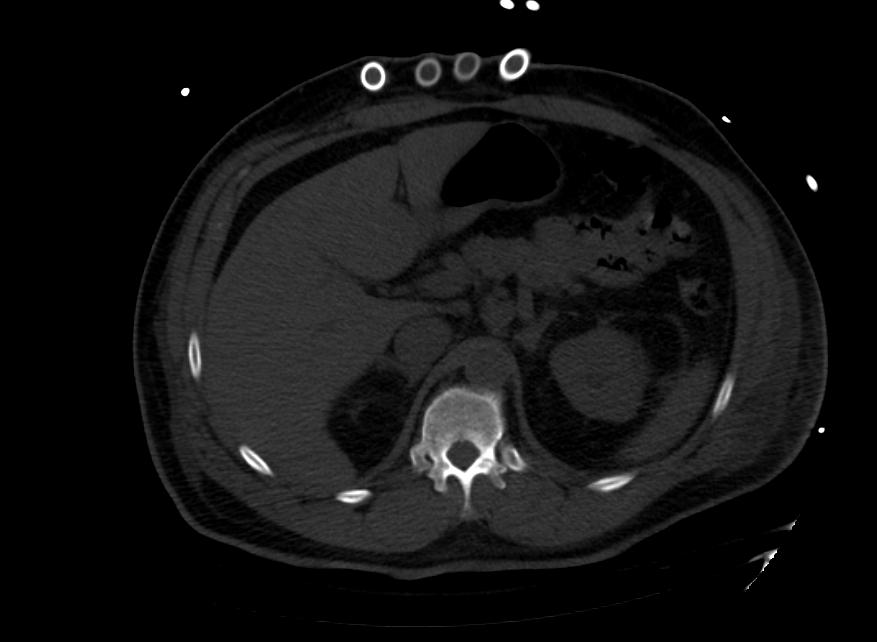
Images: Ventricular Assist Devices
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
-
Biventricular assist device
List of implantable VAD devices
This is a partial list and may never be complete
Referenced additions are welcome
| Device | Manufacturer | Type | Approval Status as at December 2007 |
|---|---|---|---|
| Novacor | World Heart | Pulsatile | Approved for use in North America, European Union and Japan |
| Heartmate | Thoratec | Pulsatile | Approved for use in North America |
| Heartmate II | Thoratec | Rotor driven continuous axial flow, ball and cup bearings. | Approved for use in European Union. FDA approval for bridge to transplant achieved November 2007. |
| Incor | Berlin Heart | Continuous flow driven by a magnetically suspended axial flow rotor. | Approved for use in European Union. |
| Jarvik 2000 | Jarvik Heart | Continuous flow, axial rotor supported by ceramic bearings | Approved for use in the European Union. Clinical trials for FDA approval are planned. |
| MicroMed DeBakey VAD | MicroMed | Continuous flow driven by axial rotor supported by ceramic bearings | Approved for use in the European Union. The child version is approved for use in children in USA. Undergoing clinical trials in USA for FDA approval. |
| VentrAssist | Ventracor | Continuous flow driven by a hydrodynamically suspended centrifugal rotor. | Approved for use in European Union and Australia. Undergoing clinical trials for FDA approval |
| MTIHeartLVAD | MiTiHeart Corporation | Continuous flow driven by a magnetically suspended centrifugal rotor. | Yet to start clinical trials. |
| C-Pulse | Sunshine Heart | Pulsatile, driven by an inflatable cuff around the aorta | Yet to start clinical trials |
| HVAD | HeartWare | Similar to Ventracor - magnetic impellor similar to BLDCmotor | undergoing clinical trials for CE, applied to start FDA US trials
|
References
- ↑ 1.0 1.1 Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK; et al. (2015). "2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention". J Am Coll Cardiol. 65 (19): e7–e26. doi:10.1016/j.jacc.2015.03.036. PMID 25861963.
- ↑ Pratt AK, Shah NS, Boyce SW (January 2014). "Left ventricular assist device management in the ICU". Crit. Care Med. 42 (1): 158–68. doi:10.1097/01.ccm.0000435675.91305.76. PMID 24240731.
- ↑ 3.0 3.1 Sutcliffe P, Connock M, Pulikottil-Jacob R, Kandala NB, Suri G, Gurung T; et al. (2013). "Clinical effectiveness and cost-effectiveness of second- and third-generation left ventricular assist devices as either bridge to transplant or alternative to transplant for adults eligible for heart transplantation: systematic review and cost-effectiveness model". Health Technol Assess. 17 (53): 1–499, v–vi. doi:10.3310/hta17530. PMC 4781283. PMID 24280231.
- ↑ https://www.openanesthesia.org/ventricular_assist_devices/
- ↑ Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH (August 2007). "Use of a continuous-flow device in patients awaiting heart transplantation". N. Engl. J. Med. 357 (9): 885–96. doi:10.1056/NEJMoa067758. PMID 17761592.
- ↑ Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Miller MA, Baldwin JT, Timothy Baldwin J, Young JB (June 2014). "Sixth INTERMACS annual report: a 10,000-patient database". J. Heart Lung Transplant. 33 (6): 555–64. doi:10.1016/j.healun.2014.04.010. PMID 24856259.
- ↑ Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH (December 2009). "Advanced heart failure treated with continuous-flow left ventricular assist device". N. Engl. J. Med. 361 (23): 2241–51. doi:10.1056/NEJMoa0909938. PMID 19920051.
- ↑ Prinzing A, Herold U, Berkefeld A, Krane M, Lange R, Voss B (August 2016). "Left ventricular assist devices-current state and perspectives". J Thorac Dis. 8 (8): E660–6. doi:10.21037/jtd.2016.07.13. PMC 4999658. PMID 27621895.
- ↑ Imamura T, Kinugawa K (October 2016). "Indication of Ventricular Assist Device Therapy in Patients with INTERMACS Profile 4-7". Ann Thorac Cardiovasc Surg. 22 (5): 271–274. doi:10.5761/atcs.ed.16-00119. PMC 5088391. PMID 27349307.
- ↑ Sayer G, Naka Y, Jorde UP (2009). "Ventricular assist device therapy". Cardiovasc Ther. 27 (2): 140–50. doi:10.1111/j.1755-5922.2009.00081.x. PMID 19426251.
- ↑ Trinquero P, Pirotte A, Gallagher LP, Iwaki KM, Beach C, Wilcox JE (September 2018). "Left Ventricular Assist Device Management in the Emergency Department". West J Emerg Med. 19 (5): 834–841. doi:10.5811/westjem.2018.5.37023. PMC 6123099. PMID 30202496.
- ↑ "Left Ventricular Assist Devices (LVAD) - StatPearls - NCBI Bookshelf".
- ↑ Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK; et al. (2015). "2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie D'intervention)". Catheter Cardiovasc Interv. 85 (7): E175–96. doi:10.1002/ccd.25720. PMID 25851050.
- ↑ Briasoulis A, Telila T, Palla M, Mercado N, Kondur A, Grines C; et al. (2016). "Meta-Analysis of Usefulness of Percutaneous Left Ventricular Assist Devices for High-Risk Percutaneous Coronary Interventions". Am J Cardiol. 118 (3): 369–75. doi:10.1016/j.amjcard.2016.05.003. PMID 27265673.
- ↑ Shi W, Wang W, Wang K, Huang W (2019). "Percutaneous mechanical circulatory support devices in high-risk patients undergoing percutaneous coronary intervention: A meta-analysis of randomized trials". Medicine (Baltimore). 98 (37): e17107. doi:10.1097/MD.0000000000017107. PMC 6750338 Check
|pmc=value (help). PMID 31517843. - ↑ O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J; et al. (2012). "A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study". Circulation. 126 (14): 1717–27. doi:10.1161/CIRCULATIONAHA.112.098194. PMID 22935569.
- ↑ 17.0 17.1 17.2 Eisen HJ (July 2019). "Left Ventricular Assist Devices (LVADS): History, Clinical Application and Complications". Korean Circ J. 49 (7): 568–585. doi:10.4070/kcj.2019.0161. PMC 6597447 Check
|pmc=value (help). PMID 31243930. - ↑ Gordon RJ, Quagliarello B, Lowy FD (2006). "Ventricular assist device-related infections". Lancet Infect Dis. 6 (7): 426&ndash, 37.
External links
- Nader Moazami, Patrick M. McCarthy Temporary Circulatory Support
- Eugene L. Kukuy, Mehmet C. Oz, Yoshifumi Naka Long-Term Mechanical Circulatory Support - a review of the subject as at 2003.
- Health Center Online VAD
- Mayo Clinic VAD
- FDA VAD
- Life without a pulse — news story about Canadian man with VAD
- Heart Pump Design Could Give Patients New Hope — A new counter-flow heart pump developed by Queensland University of Technology
- Heart pump improves quality of life in congestive heart failure patientsA rapid review of the medical literature and specialist opinion as at December 2005
- NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE (UK) Interventional procedures overview - short-term circulatory support with left ventricular assist devices as a bridge to cardiac transplantation or recovery A rapid review of the medical literature and specialist opinion as at December 2005
- Courtney J. Gemmato,Matthew D. Forrester,Timothy J. Myers,O.H. Frazier,Denton A. Cooley Thirty-Five Years of Mechanical Circulatory Support at the Texas Heart InstituteTex Heart Inst J 2005;32:168-77