Etanercept sandbox
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
SERIOUS INFECTIONS AND MALIGNANCIES
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS
Increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections due to other opportunistic pathogens. (5.1) Etanercept should be discontinued if a patient develops a serious infection or sepsis during treatment. (5.1) Perform test for latent TB; if positive, start treatment for TB prior to starting Etanercept . (5.1) Monitor all patients for active TB during treatment, even if initial latent TB test is negative. (5.1) MALIGNANCIES Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including Etanercept . (5.3) |
Overview
Etanercept sandbox is a Tumor Necrosis Factor Blocker that is FDA approved for the {{{indicationType}}} of rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include injection site reaction, rhinitis, upper respiratory infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
General Information

Adult Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis Patients
- Dosing informatinon
- MTX, glucocorticoids, salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), or analgesics may be continued during treatment with Etanercept .
- Recommended dosage: 50 mg twice weekly
- Doses higher than 50 mg per week are not recommended.
Adult Plaque Psoriasis Patients
- Dosing informatinon
- Recommended starting dose: 50 mg twice weekly ,
- Starting doses of 25 mg or 50 mg per week were shown to be efficacious. The proportion of responders was related to Etanercept dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Etanercept in adult patients.
Non–Guideline-Supported Use
Behcet's syndrome
- Dosing information
- 25 mg twice weekly for 4 weeks[1]
Bone metastasis - Pain from metastases
- Dosing information
- 25 mg[2]
Crohn's disease
- Dosing information
- 25 mg SC twice weekly[3]
Hemophagocytic lymphohistiocytosis
- Dosing information
- Twice weekly injections (0.4 mg/kg)[4]
Hidradenitis suppurativa
- Dosing information
- 25 mg twice weekly [5]
Langerhans cell histiocytosis
- Dosing information
- 0.4 mg/kg SC twice weekly[6]
Myelosclerosis with myeloid metaplasia
- Dosing information
- 25 mg twice weekly 11877307
- 25 mg twice weekly subQ for at least 4 weeks[7]
Nephrotic syndrome
- Dosing information
- 25-mg SC injections twice weekly[8]
Pemphigoid
- Dosing information
- 25 mg subQ twice weekly [9]
Sarcoidosis
- Dosing information
- 25 mg twice weekly[10]
Sjögren's syndrome
- Dosing information
- 25 mg twice per week for 12 weeks[11]
TNF receptor-associated periodic fever syndrome (TRAPS)
- Dosing information
- 25 mg (adults) or 0.4 mg/kg (children) twice weekly[12]
Uveitis
- Dosing information
- 25 mg twice a week for 24 weeks[13]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
JIA Patients
- Dosing informatinon
- In JIA patients, glucocorticoids, NSAIDs, or analgesics may be continued during treatment with Etanercept . Higher doses of Etanercept have not been studied in pediatric patients.

Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Etanercept in pediatric patients.
Non–Guideline-Supported Use
Graft versus host disease
- Dosing information
- 0.4 mg/kg SC twice weekly [14]
Contraindications
Etanercept should not be administered to patients with sepsis.
Warnings
|
SERIOUS INFECTIONS AND MALIGNANCIES
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS
Increased risk of serious infections leading to hospitalization or death, including tuberculosis (TB), bacterial sepsis, invasive fungal infections (such as histoplasmosis), and infections due to other opportunistic pathogens. (5.1) Etanercept should be discontinued if a patient develops a serious infection or sepsis during treatment. (5.1) Perform test for latent TB; if positive, start treatment for TB prior to starting Etanercept . (5.1) Monitor all patients for active TB during treatment, even if initial latent TB test is negative. (5.1) MALIGNANCIES Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including Etanercept . (5.3) |
Serious Infections
Patients treated with Etanercept are at increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death. Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, parasitic, or other opportunistic pathogens including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis, and Tuberculosis have been reported with TNF blockers. Patients have frequently presented with disseminated rather than localized disease. Treatment with Etanercept should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants (such as corticosteroids or methotrexate), may be at greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- With chronic or recurrent infection;
- Who have been exposed to Tuberculosis;
- With a history of an opportunistic infection;
- Who have resided or traveled in areas of endemic Tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis; or
- With underlying conditions that may predispose them to infection, such as advanced or poorly controlled diabetes [see Adverse Reactions (6.1)].
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with Etanercept . Etanercept should be discontinued if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with Etanercept should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
Cases of reactivation of Tuberculosis or new Tuberculosis infections have been observed in patients receiving Etanercept , including patients who have previously received treatment for latent or active Tuberculosis. Data from clinical trials and preclinical studies suggest that the risk of reactivation of latent Tuberculosis infection is lower with Etanercept than with TNF-blocking monoclonal antibodies. Nonetheless, postmarketing cases of Tuberculosis reactivation have been reported for TNF blockers, including Etanercept . Tuberculosis has developed in patients who tested negative for latent Tuberculosis prior to initiation of therapy. Patients should be evaluated for Tuberculosis risk factors and tested for latent infection prior to initiating Etanercept and periodically during therapy. Tests for latent Tuberculosis infection may be falsely negative while on therapy with Etanercept . Treatment of latent Tuberculosis infection prior to therapy with TNF-blocking agents has been shown to reduce the risk of Tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent Tuberculosis is needed prior to initiating Etanercept , even for patients previously vaccinated with Bacille Calmette-Guerin (BCG). Anti-Tuberculosis therapy should also be considered prior to initiation of Etanercept in patients with a past history of latent or active Tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent Tuberculosis but having risk factors for Tuberculosis infection. Consultation with a physician with expertise in the treatment of Tuberculosis is recommended to aid in the decision whether initiating anti-Tuberculosis therapy is appropriate for an individual patient. Tuberculosis should be strongly considered in patients who develop a new infection during Etanercept treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of Tuberculosis, or who have had close contact with a person with active Tuberculosis.
Invasive Fungal Infections
Cases of serious and sometimes fatal fungal infections, including histoplasmosis, have been reported with TNF blockers, including Etanercept . For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric anti-fungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric anti-fungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and the risks of anti-fungal therapy. In 38 Etanercept clinical trials and 4 cohort studies in all approved indications representing 27,169 patient-years of exposure (17,696 patients) from the United States and Canada, no histoplasmosis infections were reported among patients treated with Etanercept .
Neurologic Events
Treatment with TNF-blocking agents, including Etanercept , has been associated with rare (< 0.1%) cases of new onset or exacerbation of central nervous system demyelinating disorders, some presenting with mental status changes and some associated with permanent disability, and with peripheral nervous system demyelinating disorders. Cases of transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barré syndromes, other peripheral demyelinating neuropathies, and new onset or exacerbation of seizure disorders have been reported in postmarketing experience with Etanercept therapy. Prescribers should exercise caution in considering the use of Etanercept in patients with preexisting or recent-onset central or peripheral nervous system demyelinating disorders.
Malignancies
In the controlled portions of clinical trials of TNF‑blocking agents, more cases of lymphoma have been observed among patients receiving a TNF blocker compared to control patients. During the controlled portions of Etanercept trials in adult patients with RA, AS, and PsA, 2 Lymphomas were observed among 3306 Etanercept ‑treated patients versus 0 among 1521 control patients (duration of controlled treatment ranged from 3 to 36 months). Among 6543 adult rheumatology (RA, PsA, AS) patients treated with Etanercept in controlled and uncontrolled portions of clinical trials, representing approximately 12,845 patient‑years of therapy, the observed rate of lymphoma was 0.10 cases per 100 patient‑years. This was 3‑fold higher than the rate of lymphoma expected in the general U.S. population based on the Surveillance, Epidemiology, and End Results (SEER) Database. An increased rate of lymphoma up to several-fold has been reported in the RA patient population, and may be further increased in patients with more severe disease activity. Among 4410 adult PsO patients treated with Etanercept in clinical trials up to 36 months, representing approximately 4278 patient‑years of therapy, the observed rate of lymphoma was 0.05 cases per 100 patient‑years, which is comparable to the rate in the general population. No cases were observed in Etanercept - or placebo-treated patients during the controlled portions of these trials.
Cases of acute and chronic Leukemia have been reported in association with postmarketing TNF-blocker use in rheumatoid arthritis and other indications. Even in the absence of TNF-blocker therapy, patients with rheumatoid arthritis may be at higher risk (approximately 2-fold) than the general population for the development of Leukemia. During the controlled portions of Etanercept trials, 2 cases of Leukemia were observed among 5445 (0.06 cases per 100 patient-years) Etanercept -treated patients versus 0 among 2890 (0%) control patients (duration of controlled treatment ranged from 3 to 48 months). Among 15,401 patients treated with Etanercept in controlled and open portions of clinical trials representing approximately 23,325 patient-years of therapy, the observed rate of Leukemia was 0.03 cases per 100 patient-years.
Other Malignancies
Information is available from 10,953 adult patients with 17,123 patient-years and 696 pediatric patients with 1282 patient-years of experience across 45 Etanercept clinical studies. For malignancies other than lymphoma and non-melanoma skin cancer, there was no difference in exposure-adjusted rates between the Etanercept and control arms in the controlled portions of clinical studies for all indications. Analysis of the malignancy rate in combined controlled and uncontrolled portions of studies has demonstrated that types and rates are similar to what is expected in the general U.S. population based on the SEER database and suggests no increase in rates over time. Whether treatment with Etanercept might influence the development and course of malignancies in adults is unknown.
Melanoma and Non-melanoma skin cancer (NMSC)
Melanoma and non-melanoma skin cancer has been reported in patients treated with TNF antagonists including etanercept. Among 15,401 patients treated with Etanercept in controlled and open portions of clinical trials representing approximately 23,325 patient-years of therapy, the observed rate of melanoma was 0.043 cases per 100 patient-years. Among 3306 adult rheumatology (RA, PsA, AS) patients treated with Etanercept in controlled clinical trials representing approximately 2669 patient‑years of therapy, the observed rate of NMSC was 0.41 cases per 100 patient‑years vs 0.37 cases per 100 patient-years among 1521 control-treated patients representing 1077 patient-years. Among 1245 adult psoriasis patients treated with Etanercept in controlled clinical trials, representing approximately 283 patient‑years of therapy, the observed rate of NMSC was 3.54 cases per 100 patient-years vs 1.28 cases per 100 patient-years among 720 control-treated patients representing 156 patient-years. Postmarketing cases of Merkel cell carcinoma have been reported very infrequently in patients treated with Etanercept . Periodic skin examinations should be considered for all patients at increased risk for skin cancer.
Pediatric Patients
Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy at ≤ 18 years of age), including Etanercept . Approximately half the cases were Lymphomas, including Hodgkin’s and non-Hodgkin’s lymphoma. The other cases represented a variety of different malignancies and included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months of therapy (range 1 to 84 months). Most of the patients were receiving concomitant immunosuppressants. These cases were reported postmarketing and are derived from a variety of sources, including registries and spontaneous postmarketing reports. In clinical trials of 1140 pediatric patients representing 1927.2 patient-years of therapy, no malignancies, including lymphoma or NMSC, have been reported.
Postmarketing Use
In global postmarketing adult and pediatric use, lymphoma and other malignancies have been reported.
Patients With Heart Failure
Two clinical trials evaluating the use of Etanercept in the treatment of heart failure were terminated early due to lack of efficacy. One of these studies suggested higher mortality in Etanercept -treated patients compared to placebo [see Adverse Reactions (6.2)]. There have been postmarketing reports of worsening of congestive heart failure (CHF), with and without identifiable precipitating factors, in patients taking Etanercept . There have also been rare (< 0.1%) reports of new onset CHF, including CHF in patients without known preexisting cardiovascular disease. Some of these patients have been under 50 years of age. Physicians should exercise caution when using Etanercept in patients who also have heart failure, and monitor patients carefully.
Hematologic Events
Rare (< 0.1%) reports of pancytopenia, including very rare (< 0.01%) reports of aplastic anemia, some with a fatal outcome, have been reported in patients treated with Etanercept . The causal relationship to Etanercept therapy remains unclear. Although no high-risk group has been identified, caution should be exercised in patients being treated with Etanercept who have a previous history of significant hematologic abnormalities. All patients should be advised to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (eg, persistent fever, bruising, bleeding, pallor) while on Etanercept . Discontinuation of Etanercept therapy should be considered in patients with confirmed significant hematologic abnormalities. Two percent of patients treated concurrently with Etanercept and Anakinra developed neutropenia (ANC < 1 x 109/L). While neutropenic, one patient developed cellulitis that resolved with antibiotic therapy.
Hepatitis B Reactivation
Reactivation of hepatitis B in patients who were previously infected with the hepatitis B virus (HBV) and had received concomitant TNF-blocking agents, including very rare cases (< 0.01%) with Etanercept , has been reported. In some instances, hepatitis B reactivation occurring in conjunction with TNF-blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to hepatitis B reactivation. Patients at risk for HBV infection should be evaluated for prior evidence of HBV infection before initiating TNF-blocker therapy. Prescribers should exercise caution in prescribing TNF blockers in patients previously infected with HBV. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF-blocker therapy to prevent HBV reactivation. Patients previously infected with HBV and require treatment with Etanercept should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop HBV reactivation, consideration should be given to stopping Etanercept and initiating anti-viral therapy with appropriate supportive treatment. The safety of resuming Etanercept therapy after HBV reactivation is controlled is not known. Therefore, prescribers should weigh the risks and benefits when considering resumption of therapy in this situation.
Allergic Reactions
Allergic reactions associated with administration of Etanercept during clinical trials have been reported in < 2% of patients. If an anaphylactic reaction or other serious allergic reaction occurs, administration of Etanercept should be discontinued immediately and appropriate therapy initiated. Caution: The following components contain dry natural rubber (a derivative of latex), which may cause allergic reactions in individuals sensitive to latex: the needle cover of the prefilled syringe and the needle cover within the needle cap of the SureClick autoinjector.
Immunizations
Live vaccines should not be given concurrently with Etanercept . It is recommended that pediatric patients, if possible, be brought up-to-date with all immunizations in agreement with current immunization guidelines prior to initiating Etanercept therapy
Autoimmunity
Treatment with Etanercept may result in the formation of autoantibodies [see Adverse Reactions (6.1)] and, rarely (< 0.1%), in the development of a lupus-like syndrome or autoimmune hepatitis [see Adverse Reactions (6.2)], which may resolve following withdrawal of Etanercept . If a patient develops symptoms and findings suggestive of a lupus-like syndrome or autoimmune hepatitis following treatment with Etanercept , treatment should be discontinued and the patient should be carefully evaluated.
Immunosuppression
TNF mediates inflammation and modulates cellular immune responses. TNF-blocking agents, including Etanercept , affect host defenses against infections. The effect of TNF inhibition on the development and course of malignancies is not fully understood. In a study of 49 patients with RA treated with Etanercept , there was no evidence of depression of delayed‑type hypersensitivity, depression of immunoglobulin levels, or change in enumeration of effector cell populations
Use in Wegener’s granulomatosis Patients
The use of Etanercept in patients with Wegener’s granulomatosis receiving immunosuppressive agents is not recommended. In a study of patients with Wegener’s granulomatosis, the addition of Etanercept to standard therapy (including cyclophosphamide) was associated with a higher incidence of non-cutaneous solid malignancies and was not associated with improved clinical outcomes when compared with standard therapy alone
Use with Anakinra or Abatacept
Use of Etanercept with Anakinra or Abatacept is not recommended [see Drug Interactions (7.2)].
Use in Patients with Moderate to Severe Alcoholic Hepatitis
In a study of 48 hospitalized patients treated with Etanercept or placebo for moderate to severe alcoholic hepatitis, the mortality rate in patients treated with Etanercept was similar to patients treated with placebo at 1 month but significantly higher after 6 months. Physicians should use caution when using Etanercept in patients with moderate to severe alcoholic hepatitis.
Adverse Reactions
Clinical Trials Experience
Adverse Reactions in Adult Patients with Rheumatoid Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, or Plaque Psoriasis
The data described below reflect exposure to Etanercept in 2219 adult patients with RA followed for up to 80 months, in 182 patients with PsA for up to 24 months, in 138 patients with AS for up to 6 months, and in 1204 adult patients with PsO for up to 18 months. In controlled trials, the proportion of Etanercept ‑treated patients who discontinued treatment due to adverse events was approximately 4% in the indications studied. Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not predict the rates observed in clinical practice.
Infections
Infections, including viral, bacterial, and fungal infections, have been observed in adult and pediatric patients. Infections have been noted in all body systems and have been reported in patients receiving Etanercept alone or in combination with other immunosuppressive agents. In controlled portions of trials, the types and severity of infection were similar between Etanercept and the respective control group (placebo or MTX for RA and PsA patients) in RA, PsA, AS and PsO patients. Rates of infections in RA and PsO patients are provided in Table 3 and Table 4, respectively. Infections consisted primarily of upper respiratory tract infection, sinusitis and influenza. In controlled portions of trials in RA, PsA, AS and PsO, the rates of serious infection were similar (0.8% in placebo, 3.6% in MTX, and 1.4% in Etanercept /Etanercept + MTX‑treated groups). In clinical trials in rheumatologic indications, serious infections experienced by patients have included, but are not limited to, pneumonia, cellulitis, septic arthritis, bronchitis, gastroenteritis, pyelonephritis, sepsis, abscess and osteomyelitis. In clinical trials in PsO, serious infections experienced by patients have included, but are not limited to, pneumonia, cellulitis, gastroenteritis, abscess and osteomyelitis. The rate of serious infections was not increased in open‑label extension trials and was similar to that observed in Etanercept ‑ and placebo‑treated patients from controlled trials. In 66 global clinical trials of 17,505 patients (21,015 patient-years of therapy), tuberculosis was observed in approximately 0.02% of patients. In 17,696 patients (27,169 patient-years of therapy) from 38 clinical trials and 4 cohort studies in the U.S. and Canada, tuberculosis was observed in approximately 0.006% of patients. These studies include reports of pulmonary and extrapulmonary tuberculosis
Injection Site Reactions
In placebo-controlled trials in rheumatologic indications, approximately 37% of patients treated with Etanercept developed injection site reactions. In controlled trials in patients with PsO, 15% of patients treated with Etanercept developed injection site reactions during the first 3 months of treatment. All injection site reactions were described as mild to moderate (erythema, itching, pain, swelling, bleeding, bruising) and generally did not necessitate drug discontinuation. Injection site reactions generally occurred in the first month and subsequently decreased in frequency. The mean duration of injection site reactions was 3 to 5 days. Seven percent of patients experienced redness at a previous injection site when subsequent injections were given.
Immunogenicity
Patients with RA, PsA, AS or PsO were tested at multiple time points for antibodies to etanercept. Antibodies to the TNF receptor portion or other protein components of the Etanercept drug product were detected at least once in sera of approximately 6% of adult patients with RA, PsA, AS or PsO. These antibodies were all non-neutralizing. Results from JIA patients were similar to those seen in adult RA patients treated with Etanercept . In PsO studies that evaluated the exposure of etanercept for up to 120 weeks, the percentage of patients testing positive at the assessed time points of 24, 48, 72 and 96 weeks ranged from 3.6%-8.7% and were all non-neutralizing. The percentage of patients testing positive increased with an increase in the duration of study; however, the clinical significance of this finding is unknown. No apparent correlation of antibody development to clinical response or adverse events was observed. The immunogenicity data of Etanercept beyond 120 weeks of exposure are unknown. The data reflect the percentage of patients whose test results were considered positive for antibodies to etanercept in an ELISA assay, and are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of any antibody positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to etanercept with the incidence of antibodies to other products may be misleading.
Autoantibodies
Patients with RA had serum samples tested for autoantibodies at multiple time points. In RA Studies I and II, the percentage of patients evaluated for antinuclear antibodies (ANA) who developed new positive ANA (titer ≥ 1:40) was higher in patients treated with Etanercept (11%) than in placebo‑treated patients (5%). The percentage of patients who developed new positive anti‑double‑stranded DNA antibodies was also higher by radioimmunoassay (15% of patients treated with Etanercept compared to 4% of placebo‑treated patients) and by Crithidia luciliae assay (3% of patients treated with Etanercept compared to none of placebo‑treated patients). The proportion of patients treated with Etanercept who developed anticardiolipin antibodies was similarly increased compared to placebo‑treated patients. In RA Study III, no pattern of increased autoantibody development was seen in Etanercept patients compared to MTX patients [see Warnings and Precautions (5.9)].
Other Adverse Reactions
Table 3 summarizes adverse reactions reported in adult RA patients. The types of adverse reactions seen in patients with PsA or AS were similar to the types of adverse reactions seen in patients with RA.
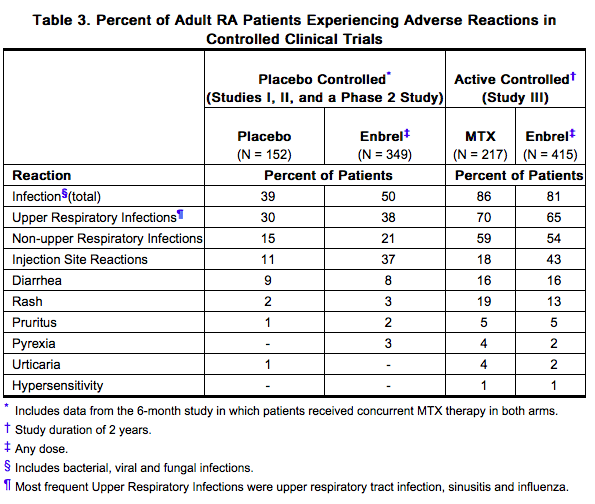
In placebo-controlled PsO trials, the percentages of patients reporting adverse reactions in the 50 mg twice a week dose group were similar to those observed in the 25 mg twice a week dose group or placebo group.
Table 4 summarizes adverse reactions reported in adult PsO patients from Studies I and II.

Adverse Reactions in Pediatric Patients
In general, the adverse reactions in pediatric patients were similar in frequency and type as those seen in adult patients [see Warnings and Precautions (5), Adverse Reactions (6), and Clinical Studies (14.2)]. The types of infections reported in pediatric patients were generally mild and consistent with those commonly seen in the general pediatric population. Two JIA patients developed varicella infection and signs and symptoms of aseptic meningitis, which resolved without sequelae. In open-label clinical studies of children with JIA, adverse reactions reported in those ages 2 to 4 years were similar to adverse reactions reported in older children.
Postmarketing Experience
Adverse reactions have been reported during post approval use of Etanercept in adults and pediatric patients. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Etanercept exposure. Adverse reactions are listed by body system below:

Opportunistic infections, including atypical mycobacterial infection, herpes zoster, aspergillosis and Pneumocystis jiroveci pneumonia, and protozoal infections have also been reported in postmarketing use.
Drug Interactions
Specific drug interaction studies have not been conducted with Etanercept .
Vaccines
Most PsA patients receiving Etanercept were able to mount effective B-cell immune responses to pneumococcal polysaccharide vaccine, but titers in aggregate were moderately lower and fewer patients had 2-fold rises in titers compared to patients not receiving Etanercept . The clinical significance of this is unknown. Patients receiving Etanercept may receive concurrent vaccinations, except for live vaccines. No data are available on the secondary transmission of infection by live vaccines in patients receiving Etanercept . Patients with a significant exposure to varicella virus should temporarily discontinue Etanercept therapy and be considered for prophylactic treatment with varicella zoster immune globulin [see Warnings and Precautions (5.8, 5.10)].
Immune-Modulating Biologic Products
In a study in which patients with active RA were treated for up to 24 weeks with concurrent Etanercept and anakinra therapy, a 7% rate of serious infections was observed, which was higher than that observed with Etanercept alone (0%) [see Warnings and Precautions (5.12)] and did not result in higher ACR response rates compared to Etanercept alone. The most common infections consisted of bacterial pneumonia (4 cases) and cellulitis (4 cases). One patient with pulmonary fibrosis and pneumonia died due to respiratory failure. Two percent of patients treated concurrently with Etanercept and anakinra developed neutropenia (ANC < 1 x 109/L). In clinical studies, concurrent administration of abatacept and Etanercept resulted in increased incidences of serious adverse events, including infections, and did not demonstrate increased clinical benefit [see Warnings and Precautions (5.12)].
Cyclophosphamide
The use of Etanercept in patients receiving concurrent cyclophosphamide therapy is not recommended [see Warnings and Precautions (5.11)].
Sulfasalazine
Patients in a clinical study who were on established therapy with sulfasalazine, to which Etanercept was added, were noted to develop a mild decrease in mean neutrophil counts in comparison to groups treated with either Etanercept or sulfasalazine alone. The clinical significance of this observation is unknown.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Pregnancy Surveillance Program
There is a Pregnancy Surveillance Program that monitors outcomes in women exposed to Etanercept during pregnancy. Women who become pregnant during Etanercept treatment are encouraged to enroll. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll.
Risk Summary
There are no adequate and well controlled studies in pregnant women. Based on limited data, etanercept concentration in cord blood at the time of delivery showed that etanercept crossed the placenta in small amounts. Developmental toxicity studies have been performed in rats and rabbits at doses ranging from 60‑ to 100‑fold higher than the human dose and have revealed no evidence of harm to the fetus due to Etanercept . Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Human Data
Three case reports showed that cord blood levels of etanercept at delivery in infants, born to mothers administered etanercept during pregnancy, were between 3 and 32% of the maternal serum level.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Etanercept sandbox in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Etanercept sandbox during labor and delivery.
Nursing Mothers
Limited data from published literature show that etanercept is present in low levels in human milk and minimally absorbed by a breastfed infant. Caution should be exercised when Etanercept is administered to a nursing woman. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for Etanercept and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition. Women who choose to continue Etanercept treatment while nursing are encouraged to enroll in Amgen’s Lactation Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll.
Pediatric Use
Etanercept has not been studied in children < 2 years of age with JIA. The safety and efficacy of Etanercept in pediatric patients with PsO have not been studied. Rare (< 0.1%) cases of IBD have been reported in JIA patients receiving Etanercept , which is not effective for the treatment of IBD [see Adverse Reactions (6.2)]. The clinical significance of infant exposure to Etanercept in utero is unknown. The safety of administering live or live-attenuated vaccines in exposed infants is unknown. Risks and benefits should be considered prior to administering live or live-attenuated vaccines to exposed infants.
Geriatic Use
A total of 480 RA patients ages 65 years or older have been studied in clinical trials. In PsO randomized clinical trials, a total of 138 out of 1965 patients treated with Etanercept or placebo were age 65 or older. No overall differences in safety or effectiveness were observed between these patients and younger patients, but the number of geriatric PsO patients is too small to determine whether they respond differently from younger patients. Because there is a higher incidence of infections in the elderly population in general, caution should be used in treating the elderly.
Gender
There is no FDA guidance on the use of Etanercept sandbox with respect to specific gender populations.
Race
There is no FDA guidance on the use of Etanercept sandbox with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Etanercept sandbox in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Etanercept sandbox in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Etanercept sandbox in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Etanercept sandbox in patients who are immunocompromised.
Use in Diabetics
There have been reports of hypoglycemia following initiation of Etanercept therapy in patients receiving medication for diabetes, necessitating a reduction in anti-diabetic medication in some of these patients.
Administration and Monitoring
Administration
Etanercept is intended for use under the guidance and supervision of a physician. Patients may self-inject when deemed appropriate and if they receive medical follow-up, as necessary. Patients should not self-administer until they receive proper training in how to prepare and administer the correct dose. The Etanercept (etanercept) “Instructions for Use” insert for each presentation contains more detailed instructions on the preparation of Etanercept .
Preparation of Etanercept Using the Single-use Prefilled Syringe or Single-use Prefilled SureClick Autoinjector
For a more comfortable injection, leave Etanercept at room temperature for about 15 to 30 minutes before injecting. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. There may be small white particles of protein in the solution. This is not unusual for proteinaceous solutions. The solution should not be used if discolored or cloudy, or if foreign particulate matter is present. When using the Etanercept single-use prefilled syringe, check to see if the amount of liquid in the prefilled syringe falls between the two purple fill level indicator lines on the syringe. If the syringe does not have the right amount of liquid, DO NOT USE THAT SYRINGE.
Preparation of Etanercept Using the Multiple-use Vial
Etanercept should be reconstituted aseptically with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (0.9% benzyl alcohol), giving a solution of 1.0 mL containing 25 mg of Etanercept . A vial adapter is supplied for use when reconstituting the lyophilized powder. However, the vial adapter should not be used if multiple doses are going to be withdrawn from the vial. If the vial will be used for multiple doses, a 25‑gauge needle should be used for reconstituting and withdrawing Etanercept , and the supplied “Mixing Date:” sticker should be attached to the vial and the date of reconstitution entered. Reconstituted solution must be refrigerated at 36°F to 46°F (2°C to 8°C) and used within 14 days. Discard reconstituted solution after 14 days because product stability and sterility cannot be assured after 14 days. DO NOT store reconstituted Etanercept solution at room temperature. For a more comfortable injection, leave the Etanercept dose tray at room temperature for about 15 to 30 minutes before injecting. If using the vial adapter, twist the vial adapter onto the diluent syringe. Then, place the vial adapter over the Etanercept vial and insert the vial adapter into the vial stopper. Push down on the plunger to inject the diluent into the Etanercept vial. If using a 25‑gauge needle to reconstitute and withdraw Etanercept , the diluent should be injected very slowly into the Etanercept vial. It is normal for some foaming to occur. Keeping the diluent syringe in place, gently swirl the contents of the Etanercept vial during dissolution. To avoid excessive foaming, do not shake or vigorously agitate. Generally, dissolution of Etanercept takes less than 10 minutes. Do not use the solution if discolored or cloudy, or if particulate matter remains. Withdraw the correct dose of reconstituted solution into the syringe. Some foam or bubbles may remain in the vial. Remove the syringe from the vial adapter or remove the 25‑gauge needle from the syringe. Attach a 27‑gauge needle to inject Etanercept . The contents of one vial of Etanercept solution should not be mixed with, or transferred into, the contents of another vial of Etanercept . No other medications should be added to solutions containing Etanercept , and do not reconstitute Etanercept with other diluents. Do not filter reconstituted solution during preparation or administration.
Monitoring
Prior to initiating Etanercept and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
Toxicology studies have been performed in monkeys at doses up to 30 times the human dose with no evidence of dose-limiting toxicities. No dose-limiting toxicities have been observed during clinical trials of Etanercept . Single IV doses up to 60 mg/m2 (approximately twice the recommended dose) have been administered to healthy volunteers in an endotoxemia study without evidence of dose-limiting toxicities.
Pharmacology
| Template:Px | |
Etanercept sandbox
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 51234.9 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 58–76% (SC) |
| Metabolism | Reticuloendothelial system (speculative) |
| Half life | 70–132 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
S4 (Au), POM (UK), ℞-only (U.S.) |
| Routes | Subcutaneous |
Mechanism of Action
TNF is a naturally occurring cytokine that is involved in normal inflammatory and immune responses. It plays an important role in the inflammatory processes of RA, polyarticular JIA, PsA, and AS and the resulting joint pathology. In addition, TNF plays a role in the inflammatory process of PsO. Elevated levels of TNF are found in involved tissues and fluids of patients with RA, JIA, PsA, AS, and PsO. Two distinct receptors for TNF (TNFRs), a 55 kilodalton protein (p55) and a 75 kilodalton protein (p75), exist naturally as monomeric molecules on cell surfaces and in soluble forms. Biological activity of TNF is dependent upon binding to either cell surface TNFR. Etanercept is a dimeric soluble form of the p75 TNF receptor that can bind TNF molecules. Etanercept inhibits binding of TNF-α and TNF-β (lymphotoxin alpha [LT-α]) to cell surface TNFRs, rendering TNF biologically inactive. In in vitro studies, large complexes of etanercept with TNF-α were not detected and cells expressing transmembrane TNF (that binds Etanercept ) are not lysed in the presence or absence of complement.
Structure
Etanercept (etanercept) is a dimeric fusion protein consisting of the extracellular ligand-binding portion of the human 75 kilodalton (p75) tumor necrosis factor receptor (TNFR) linked to the Fc portion of human IgG1. The Fc component of etanercept contains the CH2 domain, the CH3 domain and hinge region, but not the CH1 domain of IgG1. Etanercept is produced by recombinant DNA technology in a Chinese hamster ovary (CHO) mammalian cell expression system. It consists of 934 amino acids and has an apparent molecular weight of approximately 150 kilodaltons. The solution of Etanercept in the single-use prefilled syringe and the single-use prefilled SureClick autoinjector is clear and colorless, sterile, preservative-free, and is formulated at pH 6.3 ± 0.2. Etanercept is also supplied in a multiple-use vial as a sterile, white, preservative-free, lyophilized powder. Reconstitution with 1 mL of the supplied Sterile Bacteriostatic Water for Injection, USP (containing 0.9% benzyl alcohol) yields a multiple-use, clear, and colorless solution with a pH of 7.4 ± 0.3.

Pharmacodynamics
Etanercept can modulate biological responses that are induced or regulated by TNF, including expression of adhesion molecules responsible for leukocyte migration (eg, E-selectin, and to a lesser extent, intercellular adhesion molecule-1 [ICAM-1]), serum levels of cytokines (eg, IL-6), and serum levels of matrix metalloproteinase-3 (MMP-3 or stromelysin). Etanercept has been shown to affect several animal models of inflammation, including murine collagen-induced arthritis.
Pharmacokinetics
After administration of 25 mg of Etanercept by a single SC injection to 25 patients with RA, a mean ± standard deviation half‑life of 102 ± 30 hours was observed with a clearance of 160 ± 80 mL/hr. A maximum serum concentration (Cmax) of 1.1 ± 0.6 mcg/mL and time to Cmax of 69 ± 34 hours was observed in these patients following a single 25 mg dose. After 6 months of twice weekly 25 mg doses in these same RA patients, the mean Cmax was 2.4 ± 1.0 mcg/mL (N = 23). Patients exhibited a 2‑ to 7‑fold increase in peak serum concentrations and approximately 4‑fold increase in AUC0‑72 hr (range 1- to 17-fold) with repeated dosing. Serum concentrations in patients with RA have not been measured for periods of dosing that exceed 6 months. The pharmacokinetic parameters in patients with PsO were similar to those seen in patients with RA. In another study, serum concentration profiles at steady state were comparable among patients with RA treated with 50 mg Etanercept once weekly and those treated with 25 mg Etanercept twice weekly. The mean (± standard deviation) Cmax, Cmin, and partial AUC were 2.4 ± 1.5 mcg/mL, 1.2 ± 0.7 mcg/mL, and 297 ± 166 mcg•h/mL, respectively, for patients treated with 50 mg Etanercept once weekly (N = 21); and 2.6 ± 1.2 mcg/mL, 1.4 ± 0.7 mcg/mL, and 316 ± 135 mcg•h/mL for patients treated with 25 mg Etanercept twice weekly (N = 16). Patients with JIA (ages 4 to 17 years) were administered 0.4 mg/kg of Etanercept twice weekly (up to a maximum dose of 50 mg per week) for up to 18 weeks. The mean serum concentration after repeated SC dosing was 2.1 mcg/mL, with a range of 0.7 to 4.3 mcg/mL. Limited data suggest that the clearance of etanercept is reduced slightly in children ages 4 to 8 years. Population pharmacokinetic analyses predict that the pharmacokinetic differences between the regimens of 0.4 mg/kg twice weekly and 0.8 mg/kg once weekly in JIA patients are of the same magnitude as the differences observed between twice weekly and weekly regimens in adult RA patients. In clinical studies with Etanercept , pharmacokinetic parameters were not different between men and women and did not vary with age in adult patients. The pharmacokinetics of etanercept were unaltered by concomitant MTX in RA patients. No formal pharmacokinetic studies have been conducted to examine the effects of renal or hepatic impairment on etanercept disposition.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been conducted to evaluate the carcinogenic potential of etanercept or its effect on fertility. Mutagenesis studies were conducted in vitro and in vivo, and no evidence of mutagenic activity was observed.
Clinical Studies
Adult Rheumatoid Arthritis
The safety and efficacy of Etanercept were assessed in four randomized, double-blind, controlled studies. The results of all four trials were expressed in percentage of patients with improvement in RA using ACR response criteria. Study I evaluated 234 patients with active RA who were ≥ 18 years old, had failed therapy with at least one but no more than four disease-modifying antirheumatic drugs (DMARDs) (eg, hydroxychloroquine, oral or injectable gold, MTX, azathioprine, D-penicillamine, sulfasalazine), and had ≥ 12 tender joints, ≥ 10 swollen joints, and either erythrocyte sedimentation rate (ESR) ≥ 28 mm/hr, C-reactive protein (CRP) > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg Etanercept or placebo were administered SC twice a week for 6 consecutive months. Study II evaluated 89 patients and had similar inclusion criteria to Study I except that patients in Study II had additionally received MTX for at least 6 months with a stable dose (12.5 to 25 mg/week) for at least 4 weeks and they had at least 6 tender or painful joints. Patients in Study II received a dose of 25 mg Etanercept or placebo SC twice a week for 6 months in addition to their stable MTX dose. Study III compared the efficacy of Etanercept to MTX in patients with active RA. This study evaluated 632 patients who were ≥ 18 years old with early (≤ 3 years disease duration) active RA, had never received treatment with MTX, and had ≥ 12 tender joints, ≥ 10 swollen joints, and either ESR ≥ 28 mm/hr, CRP > 2.0 mg/dL, or morning stiffness for ≥ 45 minutes. Doses of 10 mg or 25 mg Etanercept were administered SC twice a week for 12 consecutive months. The study was unblinded after all patients had completed at least 12 months (and a median of 17.3 months) of therapy. The majority of patients remained in the study on the treatment to which they were randomized through 2 years, after which they entered an extension study and received open-label 25 mg Etanercept . MTX tablets (escalated from 7.5 mg/week to a maximum of 20 mg/week over the first 8 weeks of the trial) or placebo tablets were given once a week on the same day as the injection of placebo or Etanercept doses, respectively. Study IV evaluated 682 adult patients with active RA of 6 months to 20 years duration (mean of 7 years) who had an inadequate response to at least one DMARD other than MTX. Forty-three percent of patients had previously received MTX for a mean of 2 years prior to the trial at a mean dose of 12.9 mg. Patients were excluded from this study if MTX had been discontinued for lack of efficacy or for safety considerations. The patient baseline characteristics were similar to those of patients in Study I. Patients were randomized to MTX alone (7.5 to 20 mg weekly, dose escalated as described for Study III; median dose 20 mg), Etanercept alone (25 mg twice weekly), or the combination of Etanercept and MTX initiated concurrently (at the same doses as above). The study evaluated ACR response, Sharp radiographic score, and safety.
Clinical Response
A higher percentage of patients treated with Etanercept and Etanercept in combination with MTX achieved ACR 20, ACR 50, and ACR 70 responses and Major Clinical Responses than in the comparison groups. The results of Studies I, II, and III are summarized in Table 6. The results of Study IV are summarized in Table 7.


The time course for ACR 20 response rates for patients receiving placebo or 25 mg Etanercept in Studies I and II is summarized in Figure 1. The time course of responses to Etanercept in Study III was similar.

Among patients receiving Etanercept , the clinical responses generally appeared within 1 to 2 weeks after initiation of therapy and nearly always occurred by 3 months. A dose response was seen in Studies I and III: 25 mg Etanercept was more effective than 10 mg (10 mg was not evaluated in Study II). Etanercept was significantly better than placebo in all components of the ACR criteria as well as other measures of RA disease activity not included in the ACR response criteria, such as morning stiffness. In Study III, ACR response rates and improvement in all the individual ACR response criteria were maintained through 24 months of Etanercept therapy. Over the 2‑year study, 23% of Etanercept patients achieved a major clinical response, defined as maintenance of an ACR 70 response over a 6‑month period. The results of the components of the ACR response criteria for Study I are shown in Table 8. Similar results were observed for Etanercept ‑treated patients in Studies II and III.

After discontinuation of Etanercept , symptoms of arthritis generally returned within a month. Reintroduction of treatment with Etanercept after discontinuations of up to 18 months resulted in the same magnitudes of response as in patients who received Etanercept without interruption of therapy, based on results of open‑label studies. Continued durable responses were seen for over 60 months in open‑label extension treatment trials when patients received Etanercept without interruption. A substantial number of patients who initially received concomitant MTX or corticosteroids were able to reduce their doses or discontinue these concomitant therapies while maintaining their clinical responses.
Physical Function Response
In Studies I, II, and III, physical function and disability were assessed using the Health Assessment Questionnaire (HAQ). Additionally, in Study III, patients were administered the SF‑36 Health Survey. In Studies I and II, patients treated with 25 mg Etanercept twice weekly showed greater improvement from baseline in the HAQ score beginning in month 1 through month 6 in comparison to placebo (p < 0.001) for the HAQ disability domain (where 0 = none and 3 = severe). In Study I, the mean improvement in the HAQ score from baseline to month 6 was 0.6 (from 1.6 to 1.0) for the 25 mg Etanercept group and 0 (from 1.7 to 1.7) for the placebo group. In Study II, the mean improvement from baseline to month 6 was 0.6 (from 1.5 to 0.9) for the Etanercept /MTX group and 0.2 (from 1.3 to 1.2) for the placebo/MTX group. In Study III, the mean improvement in the HAQ score from baseline to month 6 was 0.7 (from 1.5 to 0.7) for 25 mg Etanercept twice weekly. All subdomains of the HAQ in Studies I and III were improved in patients treated with Etanercept . In Study III, patients treated with 25 mg Etanercept twice weekly showed greater improvement from baseline in SF‑36 physical component summary score compared to Etanercept 10 mg twice weekly and no worsening in the SF‑36 mental component summary score. In open‑label Etanercept studies, improvements in physical function and disability measures have been maintained for up to 4 years. In Study IV, median HAQ scores improved from baseline levels of 1.8, 1.8, and 1.8 to 1.1, 1.0, and 0.6 at 12 months in the MTX, Etanercept , and Etanercept /MTX combination treatment groups, respectively (combination versus both MTX and Etanercept , p < 0.01). Twenty-nine percent of patients in the MTX alone treatment group had an improvement of HAQ of at least 1 unit versus 40% and 51% in the Etanercept alone and the Etanercept /MTX combination treatment groups, respectively.
Radiographic Response
In Study III, structural joint damage was assessed radiographically and expressed as change in Total Sharp Score (TSS) and its components, the erosion score and joint space narrowing (JSN) score. Radiographs of hands/wrists and forefeet were obtained at baseline, 6 months, 12 months, and 24 months and scored by readers who were unaware of treatment group. The results are shown in Table 9. A significant difference for change in erosion score was observed at 6 months and maintained at 12 months.

Patients continued on the therapy to which they were randomized for the second year of Study III. Seventy-two percent of patients had x-rays obtained at 24 months. Compared to the patients in the MTX group, greater inhibition of progression in TSS and erosion score was seen in the 25 mg Etanercept group, and, in addition, less progression was noted in the JSN score. In the open-label extension of Study III, 48% of the original patients treated with 25 mg Etanercept have been evaluated radiographically at 5 years. Patients had continued inhibition of structural damage, as measured by the TSS, and 55% of them had no progression of structural damage. Patients originally treated with MTX had further reduction in radiographic progression once they began treatment with Etanercept . In Study IV, less radiographic progression (TSS) was observed with Etanercept in combination with MTX compared with Etanercept alone or MTX alone at month 12 (Table 10). In the MTX treatment group, 55% of patients experienced no radiographic progression (TSS change ≤ 0.0) at 12 months compared to 63% and 76% in the Etanercept alone and the Etanercept /MTX combination treatment groups, respectively.

Once Weekly Dosing
The safety and efficacy of 50 mg Etanercept (two 25 mg SC injections) administered once weekly were evaluated in a double‑blind, placebo‑controlled study of 420 patients with active RA. Fifty‑three patients received placebo, 214 patients received 50 mg Etanercept once weekly, and 153 patients received 25 mg Etanercept twice weekly. The safety and efficacy profiles of the two Etanercept treatment groups were similar.
Polyarticular Juvenile Idiopathic Arthritis (JIA)
The safety and efficacy of Etanercept were assessed in a 2-part study in 69 children with polyarticular JIA who had a variety of JIA onset types. Patients ages 2 to 17 years with moderately to severely active polyarticular JIA refractory to or intolerant of MTX were enrolled; patients remained on a stable dose of a single nonsteroidal anti-inflammatory drug and/or prednisone (≤ 0.2 mg/kg/day or 10 mg maximum). In part 1, all patients received 0.4 mg/kg (maximum 25 mg per dose) Etanercept SC twice weekly. In part 2, patients with a clinical response at day 90 were randomized to remain on Etanercept or receive placebo for 4 months and assessed for disease flare. Responses were measured using the JIA Definition of Improvement (DOI), defined as ≥ 30% improvement in at least three of six and ≥ 30% worsening in no more than one of the six JIA core set criteria, including active joint count, limitation of motion, physician and patient/parent global assessments, functional assessment, and ESR. Disease flare was defined as a ≥ 30% worsening in three of the six JIA core set criteria and ≥ 30% improvement in not more than one of the six JIA core set criteria and a minimum of two active joints. In part 1 of the study, 51 of 69 (74%) patients demonstrated a clinical response and entered part 2. In part 2, 6 of 25 (24%) patients remaining on Etanercept experienced a disease flare compared to 20 of 26 (77%) patients receiving placebo (p = 0.007). From the start of part 2, the median time to flare was ≥ 116 days for patients who received Etanercept and 28 days for patients who received placebo. Each component of the JIA core set criteria worsened in the arm that received placebo and remained stable or improved in the arm that continued on Etanercept . The data suggested the possibility of a higher flare rate among those patients with a higher baseline ESR. Of patients who demonstrated a clinical response at 90 days and entered part 2 of the study, some of the patients remaining on Etanercept continued to improve from month 3 through month 7, while those who received placebo did not improve. The majority of JIA patients who developed a disease flare in part 2 and reintroduced Etanercept treatment up to 4 months after discontinuation re-responded to Etanercept therapy in open-label studies. Most of the responding patients who continued Etanercept therapy without interruption have maintained responses for up to 48 months. Studies have not been done in patients with polyarticular JIA to assess the effects of continued Etanercept therapy in patients who do not respond within 3 months of initiating Etanercept therapy, or to assess the combination of Etanercept with MTX.
Psoriatic Arthritis
The safety and efficacy of Etanercept were assessed in a randomized, double-blind, placebo-controlled study in 205 patients with PsA. Patients were between 18 and 70 years of age and had active PsA (≥ 3 swollen joints and ≥ 3 tender joints) in one or more of the following forms: (1) distal interphalangeal (DIP) involvement (N = 104); (2) polyarticular arthritis (absence of rheumatoid nodules and presence of psoriasis; N = 173); (3) arthritis mutilans (N = 3); (4) asymmetric psoriatic arthritis (N = 81); or (5) ankylosing spondylitis-like (N = 7). Patients also had plaque psoriasis with a qualifying target lesion ≥ 2 cm in diameter. Patients on MTX therapy at enrollment (stable for ≥ 2 months) could continue at a stable dose of ≤ 25 mg/week MTX. Doses of 25 mg Etanercept or placebo were administered SC twice a week during the initial 6-month double-blind period of the study. Patients continued to receive blinded therapy in an up to 6-month maintenance period until all patients had completed the controlled period. Following this, patients received open-label 25 mg Etanercept twice a week in a 12-month extension period. Compared to placebo, treatment with Etanercept resulted in significant improvements in measures of disease activity (Table 11).

Among patients with PsA who received Etanercept , the clinical responses were apparent at the time of the first visit (4 weeks) and were maintained through 6 months of therapy. Responses were similar in patients who were or were not receiving concomitant MTX therapy at baseline. At 6 months, the ACR 20/50/70 responses were achieved by 50%, 37%, and 9%, respectively, of patients receiving Etanercept , compared to 13%, 4%, and 1%, respectively, of patients receiving placebo. Similar responses were seen in patients with each of the subtypes of PsA, although few patients were enrolled with the arthritis mutilans and ankylosing spondylitis-like subtypes. The results of this study were similar to those seen in an earlier single-center, randomized, placebo-controlled study of 60 patients with PsA. The skin lesions of psoriasis were also improved with Etanercept , relative to placebo, as measured by percentages of patients achieving improvements in the Psoriasis Area and Severity Index (PASI). Responses increased over time, and at 6 months, the proportions of patients achieving a 50% or 75% improvement in the PASI were 47% and 23%, respectively, in the Etanercept group (N = 66), compared to 18% and 3%, respectively, in the placebo group (N = 62). Responses were similar in patients who were or were not receiving concomitant MTX therapy at baseline.
Radiographic Response
Radiographic changes were also assessed in the PsA study. Radiographs of hands and wrists were obtained at baseline and months 6, 12, and 24. A modified Total Sharp Score (TSS), which included distal interphalangeal joints (ie, not identical to the modified TSS used for RA) was used by readers blinded to treatment group to assess the radiographs. Some radiographic features specific to PsA (eg, pencil-and-cup deformity, joint space widening, gross osteolysis, and ankylosis) were included in the scoring system, but others (eg, phalangeal tuft resorption, juxta-articular and shaft periostitis) were not. Most patients showed little or no change in the modified TSS during this 24-month study (median change of 0 in both patients who initially received Etanercept or placebo). More placebo-treated patients experienced larger magnitudes of radiographic worsening (increased TSS) compared to Etanercept treatment during the controlled period of the study. At 12 months, in an exploratory analysis, 12% (12 of 104) of placebo patients compared to none of the 101 Etanercept -treated patients had increases of 3 points or more in TSS. Inhibition of radiographic progression was maintained in patients who continued on Etanercept during the second year. Of the patients with 1-year and 2-year x-rays, 3% (2 of 71) had increases of 3 points or more in TSS at 1 and 2 years.
Physical Function Response
In the PsA study, physical function and disability were assessed using the HAQ Disability Index (HAQ-DI) and the SF-36 Health Survey. Patients treated with 25 mg Etanercept twice weekly showed greater improvement from baseline in the HAQ-DI score (mean decreases of 54% at both months 3 and 6) in comparison to placebo (mean decreases of 6% at both months 3 and 6) (p < 0.001). At months 3 and 6, patients treated with Etanercept showed greater improvement from baseline in the SF-36 physical component summary score compared to patients treated with placebo, and no worsening in the SF-36 mental component summary score. Improvements in physical function and disability measures were maintained for up to 2 years through the open-label portion of the study.
Ankylosing Spondylitis
The safety and efficacy of Etanercept were assessed in a randomized, double-blind, placebo-controlled study in 277 patients with active AS. Patients were between 18 and 70 years of age and had AS as defined by the modified New York Criteria for Ankylosing Spondylitis. Patients were to have evidence of active disease based on values of ≥ 30 on a 0-100 unit Visual Analog Scale (VAS) for the average of morning stiffness duration and intensity, and two of the following three other parameters: a) patient global assessment, b) average of nocturnal and total back pain, and c) the average score on the Bath Ankylosing Spondylitis Functional Index (BASFI). Patients with complete ankylosis of the spine were excluded from study participation. Patients taking hydroxychloroquine, sulfasalazine, methotrexate, or prednisone (≤ 10 mg/day) could continue these drugs at stable doses for the duration of the study. Doses of 25 mg Etanercept or placebo were administered SC twice a week for 6 months. The primary measure of efficacy was a 20% improvement in the Assessment in Ankylosing Spondylitis (ASAS) response criteria. Compared to placebo, treatment with Etanercept resulted in improvements in the ASAS and other measures of disease activity (Figure 2 and Table 12).

At 12 weeks, the ASAS 20/50/70 responses were achieved by 60%, 45%, and 29%, respectively, of patients receiving Etanercept , compared to 27%, 13%, and 7%, respectively, of patients receiving placebo (p ≤ 0.0001, Etanercept vs placebo). Similar responses were seen at week 24. Responses were similar between those patients receiving concomitant therapies at baseline and those who were not. The results of this study were similar to those seen in a single-center, randomized, placebo-controlled study of 40 patients and a multicenter, randomized, placebo-controlled study of 84 patients with AS.

Plaque Psoriasis
The safety and efficacy of Etanercept were assessed in two randomized, double-blind, placebo-controlled studies in adults with chronic stable PsO involving ≥ 10% of the body surface area, a minimum Psoriasis Area and Severity Index (PASI) score of 10 and who had received or were candidates for systemic antipsoriatic therapy or phototherapy. Patients with guttate, erythrodermic, or pustular psoriasis and patients with severe infections within 4 weeks of screening were excluded from study. No concomitant major antipsoriatic therapies were allowed during the study. Study I evaluated 672 patients who received placebo or Etanercept SC at doses of 25 mg once a week, 25 mg twice a week, or 50 mg twice a week for 3 months. After 3 months, patients continued on blinded treatments for an additional 3 months during which time patients originally randomized to placebo began treatment with blinded Etanercept at 25 mg twice weekly (designated as placebo/Etanercept in Table 13); patients originally randomized to Etanercept continued on the originally randomized dose (designated as Etanercept /Etanercept groups in Table 13). Study II evaluated 611 patients who received placebo or Etanercept SC at doses of 25 mg or 50 mg twice a week for 3 months. After 3 months of randomized, blinded treatment, patients in all three arms began receiving open-label Etanercept at 25 mg twice weekly for 9 additional months. Response to treatment in both studies was assessed after 3 months of therapy and was defined as the proportion of patients who achieved a reduction in PASI score of at least 75% from baseline. The PASI is a composite score that takes into consideration both the fraction of body surface area affected and the nature and severity of psoriatic changes within the affected regions (induration, erythema and scaling). Other evaluated outcomes included the proportion of patients who achieved a score of “clear” or “minimal” by the Static Physician Global Assessment (sPGA) and the proportion of patients with a reduction of PASI of at least 50% from baseline. The sPGA is a 6-category scale ranging from “5 = severe” to “0 = none” indicating the physician’s overall assessment of the PsO severity focusing on induration, erythema and scaling. Treatment success of “clear” or “minimal” consisted of none or minimal elevation in plaque, up to faint red coloration in erythema and none or minimal fine scale over < 5% of the plaque. Patients in all treatment groups and in both studies had a median baseline PASI score ranging from 15 to 17, and the percentage of patients with baseline sPGA classifications ranged from 54% to 66% for moderate, 17% to 26% for marked and 1% to 5% for severe. Across all treatment groups, the percentage of patients who previously received systemic therapy for PsO ranged from 61% to 65% in Study I and 71% to 75% in Study II, and those who previously received phototherapy ranged from 44% to 50% in Study I and 72% to 73% in Study II. More patients randomized to Etanercept than placebo achieved at least a 75% reduction from baseline PASI score (PASI 75) with a dose response relationship across doses of 25 mg once a week, 25 mg twice a week, and 50 mg twice a week (Tables 13 and 14). The individual components of the PASI (induration, erythema and scaling) contributed comparably to the overall treatment-associated improvement in PASI.


Among PASI 75 achievers in both studies, the median time to PASI 50 and PASI 75 was approximately 1 month and approximately 2 months, respectively, after the start of therapy with either 25 or 50 mg twice a week. In Study I, patients who achieved PASI 75 at month 6 were entered into a study drug withdrawal and retreatment period. Following withdrawal of study drug, these patients had a median duration of PASI 75 of between 1 and 2 months. In Study I, among patients who were PASI 75 responders at 3 months, retreatment with their original blinded Etanercept dose after discontinuation of up to 5 months resulted in a similar proportion of responders as in the initial double-blind portion of the study. In Study II, most patients initially randomized to 50 mg twice a week continued in the study after month 3 and had their Etanercept dose decreased to 25 mg twice a week. Of the 91 patients who were PASI 75 responders at month 3, 70 (77%) maintained their PASI 75 response at month 6.
How Supplied
Administration of one 50 mg Etanercept prefilled syringe or one Etanercept SureClick autoinjector provides a dose equivalent to two 25 mg Etanercept prefilled syringes or two multiple-use vials of lyophilized Etanercept , when vials are reconstituted and administered as recommended.
Etanercept Single-use Prefilled Syringe and Etanercept Single-use Prefilled SureClick Autoinjector
Each Etanercept single-use prefilled syringe and Etanercept single-use prefilled SureClick autoinjector contains 50 mg/mL of etanercept in a single-dose syringe with a 27-gauge, ½-inch needle.

Etanercept Multiple-use Vial (Recommended for Weight-based Dosing)
Etanercept multiple-use vial is supplied in a carton containing four dose trays. Each dose tray contains one 25 mg vial of etanercept, one diluent syringe (1 mL Sterile Bacteriostatic Water for Injection, USP, containing 0.9% benzyl alcohol), one 27-gauge ½-inch needle, one vial adapter, and one plunger. Each carton contains four “Mixing Date:” stickers.

Storage
Etanercept Single-use Prefilled Syringe and Etanercept Single-use Prefilled SureClick Autoinjector
Etanercept should be refrigerated at 36°F to 46°F (2°C to 8°C). Do not use Etanercept beyond the expiration date stamped on the carton or barrel label. DO NOT SHAKE. Store Etanercept in the original carton to protect from light or physical damage. For convenience, storage of individual syringes or autoinjectors at room temperature for a maximum single period of 14 days is permissible, with protection from light and sources of heat. Once a syringe or autoinjector has been stored at room temperature, it should not be placed back into the refrigerator. If not used within 14 days at room temperature, the syringe or autoinjector should be discarded. Do not store Etanercept in extreme heat or cold. DO NOT FREEZE. Keep out of the reach of children.
Etanercept Multiple-use Vial (Recommended for Weight-based Dosing)
Etanercept should be refrigerated at 36°F to 46°F (2°C to 8°C). Do not use Etanercept beyond the expiration date stamped on the dose tray. DO NOT SHAKE. Store Etanercept in the original carton to protect from light or physical damage. For convenience, storage of an individual dose tray containing Etanercept multi-use vial and diluent syringe at room temperature for a maximum single period of 14 days is permissible, with protection from light, sources of heat, and humidity. Once the dose tray has been stored at room temperature, it should not be placed back into the refrigerator. If not used within 14 days at room temperature, the dose tray should be discarded. Once a vial has been reconstituted, the solution must be used immediately or may be refrigerated for up to14 days. Do not store Etanercept in extreme heat or cold. DO NOT FREEZE. Keep out of the reach of children.
Images
Drug Images
{{#ask: Page Name::Etanercept sandbox |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Etanercept sandbox |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patient Counseling
Patients should be advised of the potential benefits and risks of Etanercept . Physicians should instruct their patients to read the Medication Guide before starting Etanercept therapy and to reread each time the prescription is renewed. Infections Inform patients that Etanercept may lower the ability of their immune system to fight infections. Advise patients of the importance of contacting their doctor if they develop any symptoms of infection, tuberculosis or reactivation of hepatitis B virus infections. Other Medical Conditions Advise patients to report any signs of new or worsening medical conditions, such as central nervous system demyelinating disorders, heart failure or autoimmune disorders, such as lupus-like syndrome or autoimmune hepatitis. Counsel about the risk of lymphoma and other malignancies while receiving Etanercept . Advise patients to report any symptoms suggestive of a pancytopenia, such as bruising, bleeding, persistent fever or pallor. Allergic Reactions Advise patients to seek immediate medical attention if they experience any symptoms of severe allergic reactions. Advise latex-sensitive patients that the following components contain dry natural rubber (a derivative of latex) that may cause allergic reactions in individuals sensitive to latex: the needle cover of the prefilled syringe and the needle cover within the needle cap of the SureClick autoinjector.
Administration of Etanercept
If a patient or caregiver is to administer Etanercept , the patient or caregiver should be instructed in injection techniques and how to measure and administer the correct dose [see the Etanercept (etanercept) “Instructions for Use” insert]. The first injection should be performed under the supervision of a qualified healthcare professional. The patient’s or caregiver’s ability to inject subcutaneously should be assessed. Patients and caregivers should be instructed in the technique, as well as proper syringe and needle disposal, and be cautioned against reuse of needles and syringes. A puncture-resistant container for disposal of needles, syringes and autoinjectors should be used. If the product is intended for multiple use, additional syringes, needles and alcohol swabs will be required. Patients can be advised to call 1-888-4Etanercept (1-888-436-2735) or visit www.Etanercept .com for more information about Etanercept .
Precautions with Alcohol
In a study of 48 hospitalized patients treated with Etanercept or placebo for moderate to severe alcoholic hepatitis, the mortality rate in patients treated with Etanercept was similar to patients treated with placebo at 1 month but significantly higher after 6 months. Physicians should use caution when using Etanercept in patients with moderate to severe alcoholic hepatitis.
Brand Names
ENBREL
Look-Alike Drug Names
Etanercept - Levbid[15]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Melikoglu M, Fresko I, Mat C, Ozyazgan Y, Gogus F, Yurdakul S; et al. (2005). "Short-term trial of etanercept in Behçet's disease: a double blind, placebo controlled study". J Rheumatol. 32 (1): 98–105. PMID 15630733.
- ↑ Tobinick EL (2003). "Targeted etanercept for treatment-refractory pain due to bone metastasis: two case reports". Clin Ther. 25 (8): 2279–88. PMID 14512134.
- ↑ Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD; et al. (2001). "Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial". Gastroenterology. 121 (5): 1088–94. PMID 11677200.
- ↑ Prahalad S, Bove KE, Dickens D, Lovell DJ, Grom AA (2001). "Etanercept in the treatment of macrophage activation syndrome". J Rheumatol. 28 (9): 2120–4. PMID 11550985.
- ↑ Cusack C, Buckley C (2006). "Etanercept: effective in the management of hidradenitis suppurativa". Br J Dermatol. 154 (4): 726–9. doi:10.1111/j.1365-2133.2005.07067.x. PMID 16536817.
- ↑ Henter JI, Karlén J, Calming U, Bernstrand C, Andersson U, Fadeel B (2001). "Successful treatment of Langerhans'-cell histiocytosis with etanercept". N Engl J Med. 345 (21): 1577–8. doi:10.1056/NEJM200111223452118. PMID 11794238.
- ↑ Tsimberidou AM, Thomas D, O'Brien S, Andreeff M, Kurzrock R, Keating M; et al. (2002). "Recombinant human soluble tumor necrosis factor (TNF) receptor (p75) fusion protein Enbrel in patients with refractory hematologic malignancies". Cancer Chemother Pharmacol. 50 (3): 237–42. doi:10.1007/s00280-002-0479-6. PMID 12203106.
- ↑ Drewe E, McDermott EM, Powell RJ (2000). "Treatment of the nephrotic syndrome with etanercept in patients with the tumor necrosis factor receptor-associated periodic syndrome". N Engl J Med. 343 (14): 1044–5. doi:10.1056/NEJM200010053431412. PMID 11023397.
- ↑ Sacher C, Rubbert A, König C, Scharffetter-Kochanek K, Krieg T, Hunzelmann N (2002). "Treatment of recalcitrant cicatricial pemphigoid with the tumor necrosis factor alpha antagonist etanercept". J Am Acad Dermatol. 46 (1): 113–5. PMID 11756956.
- ↑ Khanna D, Liebling MR, Louie JS (2003). "Etanercept ameliorates sarcoidosis arthritis and skin disease". J Rheumatol. 30 (8): 1864–7. PMID 12913948.
- ↑ Zandbelt MM, de Wilde P, van Damme P, Hoyng CB, van de Putte L, van den Hoogen F (2004). "Etanercept in the treatment of patients with primary Sjögren's syndrome: a pilot study". J Rheumatol. 31 (1): 96–101. PMID 14705226.
- ↑ Bulua AC, Mogul DB, Aksentijevich I, Singh H, He DY, Muenz LR; et al. (2012). "Efficacy of etanercept in the tumor necrosis factor receptor-associated periodic syndrome: a prospective, open-label, dose-escalation study". Arthritis Rheum. 64 (3): 908–13. doi:10.1002/art.33416. PMC 3882089. PMID 22006113.
- ↑ Foster CS, Tufail F, Waheed NK, Chu D, Miserocchi E, Baltatzis S; et al. (2003). "Efficacy of etanercept in preventing relapse of uveitis controlled by methotrexate". Arch Ophthalmol. 121 (4): 437–40. doi:10.1001/archopht.121.4.437. PMID 12695239.
- ↑ Andolina M, Rabusin M, Maximova N, Di Leo G (2000). "Etanercept in graft-versus-host disease". Bone Marrow Transplant. 26 (8): 929. doi:10.1038/sj.bmt.1702638. PMID 11081399.
- ↑ "https://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_label_01.jpg
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_label_02.jpg
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_label_03.jpg
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_label_04.jpg
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_panel_01.png
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_panel_02.png
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_panel_03.png
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_panel_04.png
}}
{{#subobject:
|Label Page=Etanercept sandbox |Label Name=Etanercept_panel_05.png
}}