Bupranolol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, topical (eye drops) |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | < 10% |
| Protein binding | 76% |
| Metabolism | First pass elimination > 90% |
| Elimination half-life | 2-4 hours (plasma) |
| Excretion | > 88% renal (as carboxybupranolol) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
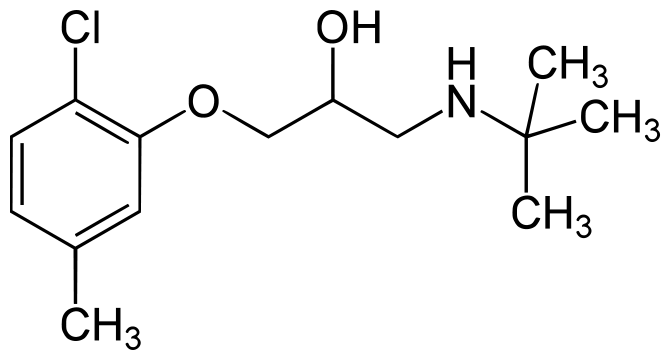
| Formula | C14H22ClNO2 |
| Molar mass | 271.78298 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Bupranolol is a non-selective beta blocker without intrinsic sympathomimetic activity (ISA), but with strong membrane stabilizing activity. Its potency is similar to propranolol.
Uses and dosage
Like other beta blockers, oral bupranolol can be used to treat hypertension and tachycardia. The initial dose is 50 mg two times a day. It can be increased to 100 mg four times a day. Bupranolol eye drops (0.05%-0.5%) are used against glaucoma.
Pharmacology
Bupranolol is quickly and completely absorbed from the gut. Over 90% undergo first-pass metabolism. Bupranolol has a plasma half life of about two to four hours, with levels never reaching 1 µg/l in therapeutic doses. The main metabolite is carboxybupranolol, 4-chloro-3-[3-(1,1-dimethylethylamino)-2-hydroxy-propyloxy]benzoic acid – that is, the methyl group at the benzene ring is oxidized to a carboxyl group –, of which 88% are eliminated renally within 24 hours.
Adverse effects, contraindications, interactions
Adverse effects, contraindications and interactions are similar to other beta blockers.
References
- Dinnendahl, V, Fricke, U, ed. (2007). Arzneistoff-Profile (in German). 2 (21 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed KEGG identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- CS1 maint: Multiple names: editors list
- CS1 maint: Unrecognized language
- Antiarrhythmic agents
- Beta blockers
- Organochlorides
- Phenol ethers
- Alcohols
- Cardiovascular Drugs
- Drugs