Darunavir contraindications: Difference between revisions
Jump to navigation
Jump to search
Gerald Chi (talk | contribs) mNo edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 6: | Line 6: | ||
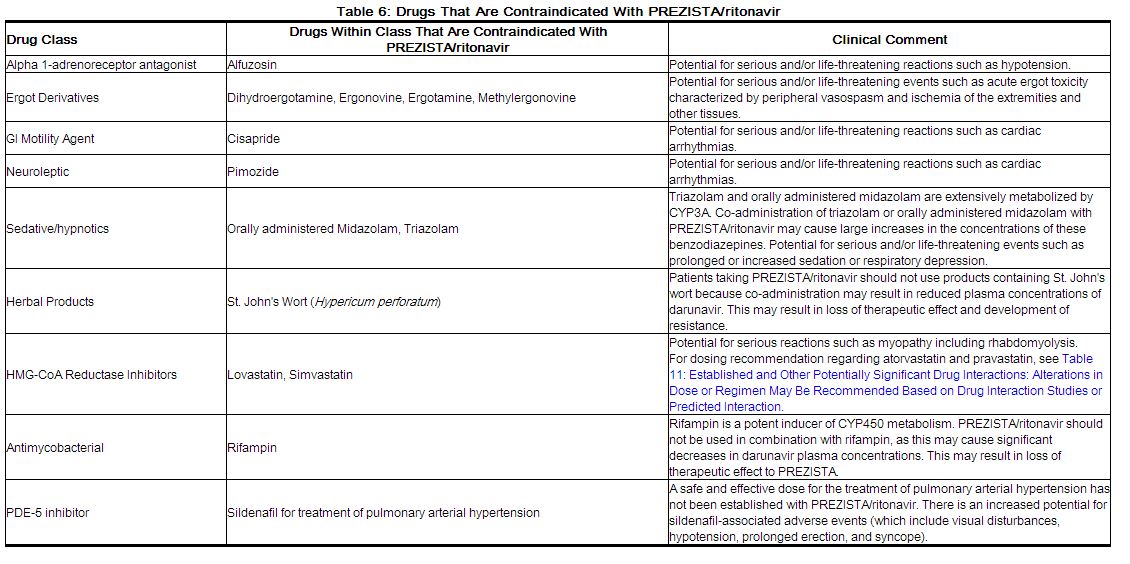
Co-administration of PREZISTA/ritonavir is contraindicated with drugs that are highly dependent on [[CYP3A]] for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). These drugs and other contraindicated drugs (which may lead to reduced efficacy of darunavir) are listed in Table 6.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = PREZISTA (DARUNAVIR) TABLET, FILM COATED PREZISTA (DARUNAVIR) SUSPENSION [JANSSEN PRODUCTS LP] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=814301f9-c990-46a5-b481-2879a521a16f#nlm34090-1 | publisher = | date = | accessdate = }}</ref> | Co-administration of PREZISTA/ritonavir is contraindicated with drugs that are highly dependent on [[CYP3A]] for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). These drugs and other contraindicated drugs (which may lead to reduced efficacy of darunavir) are listed in Table 6.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = PREZISTA (DARUNAVIR) TABLET, FILM COATED PREZISTA (DARUNAVIR) SUSPENSION [JANSSEN PRODUCTS LP] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=814301f9-c990-46a5-b481-2879a521a16f#nlm34090-1 | publisher = | date = | accessdate = }}</ref> | ||
{| | {| | ||
Latest revision as of 23:52, 31 December 2013
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Contraindications
Co-administration of PREZISTA/ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). These drugs and other contraindicated drugs (which may lead to reduced efficacy of darunavir) are listed in Table 6.[1]
 |
References
Adapted from the FDA Package Insert.