Darunavir contraindications: Difference between revisions
Jump to navigation
Jump to search
Ahmed Zaghw (talk | contribs) No edit summary |
Ahmed Zaghw (talk | contribs) |
||
| Line 8: | Line 8: | ||
{| | {| | ||
|[[File:Contraindicationsdarunavirrr.JPG| | |[[File:Contraindicationsdarunavirrr.JPG|800px|thumb]] | ||
|} | |} | ||
Revision as of 21:45, 31 December 2013
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
CONTRAINDICATIONS
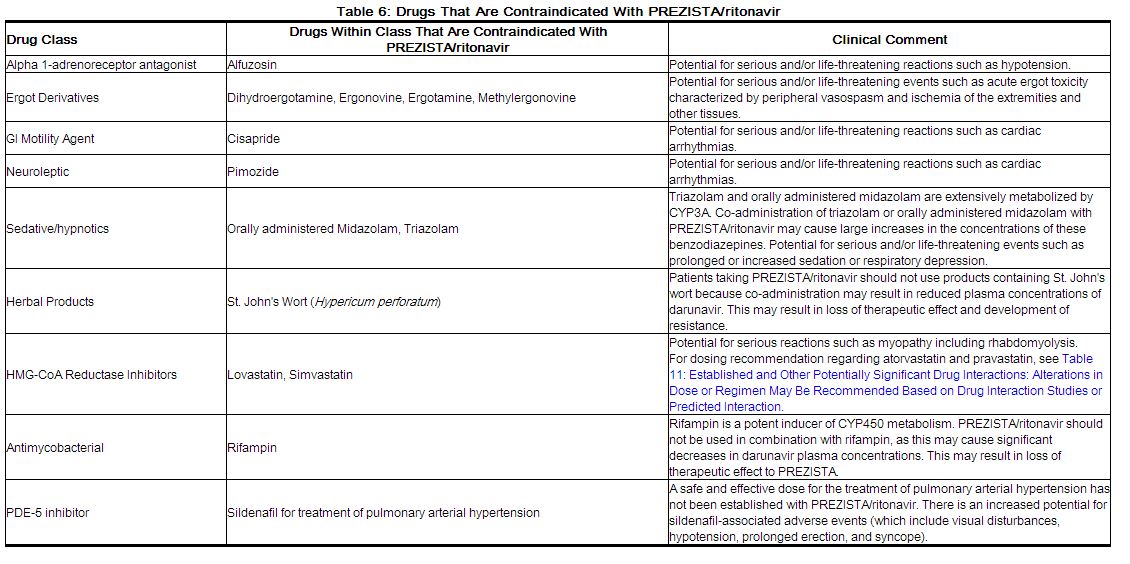
Co-administration of PREZISTA/ritonavir is contraindicated with drugs that are highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). These drugs and other contraindicated drugs (which may lead to reduced efficacy of darunavir) are listed in Table 6
 |
References
Adapted from the FDA Package Insert.