Dehydroascorbic acid: Difference between revisions
No edit summary |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
||
| Line 33: | Line 33: | ||
|} | |} | ||
{{SI}} | {{SI}} | ||
==Chemistry== | ==Chemistry== | ||
| Line 94: | Line 94: | ||
[[Category:Neurological disorders]] | [[Category:Neurological disorders]] | ||
[[Category:Causes of death]] | [[Category:Causes of death]] | ||
{{WH}} | {{WH}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
Latest revision as of 00:33, 9 August 2012
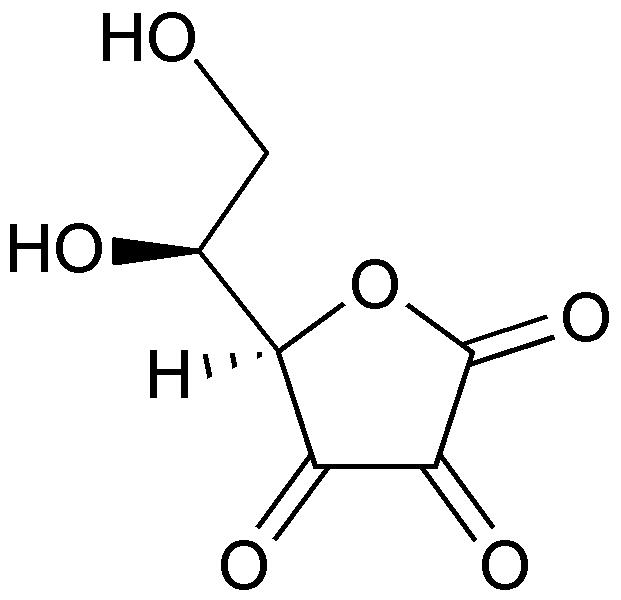
| Template:Chembox header| Dehydroascorbic acid | |
|---|---|
| |
| Systematic name | Dehydroascorbic acid |
| Chemical formula | C6H6O6 |
| Molecular mass | 174.13 g/mol |
| Density | x.xxx g/cm³ |
| Melting point | xx.x °C |
| Boiling point | xx.x °C |
| CAS number | [xx-xx-xx] |
| SMILES | xxxxx |
| Template:Chembox header | Disclaimer and references | |
Chemistry
Dehydroascorbic acid (DHA) is an oxidized form of ascorbic acid. It is actively imported into the endoplasmic reticulum of cells and generates the oxidative potential found there. Protein disulfide isomerases are known to reduce DHA back to ascorbic acid, oxidizing their disulfide bonds in the process. Therefore L-dehydroascorbic acid is a vitamin C compound much like L-ascorbic acid. Oxidized forms of esterified ascorbic acids can be numbered at C(5) or C(6) atoms and the (free) chemical radical semi-dehydroascorbate or semidehydro ascorbic acid (SDA) to the group of dehydroascorbic acids.
Physiological Significance
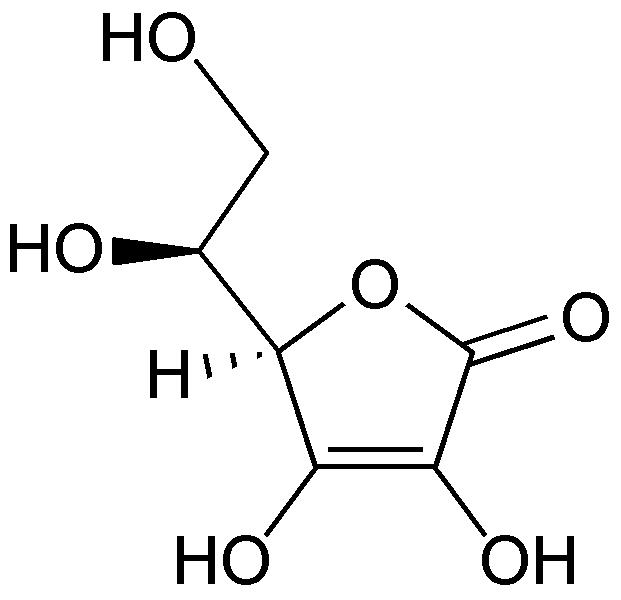 |
 |
(reduced form of Vitamin C)
Bottom: dehydroascorbic acid
(oxidized form of Vitamin C)
General significance
Although there exists a transporter for Vitamin C, it is mainly present in specialized cells, whereas the glucose transporters, most notably GLUT1, ensure in most cells of the body the transport of vitamin C (in its oxidized form, DHA)[1] where it is required as an enzyme cofactor and an intracellular antioxidant, after it is recycled back to ascorbic acid (see Transport to mitochondria).
Transport to mitochondria
Vitamin C accumulates in mitochondria, where most of the free radicals are produced, by entering through the glucose transporters, GLUT1. It is the oxidized form (dehydroascorbic acid) that is transported by GLUT1, not the reduced form (ascorbic acid). Ascorbic acid protects the mitochondrial genome and membrane.[1]
Transport to the brain
Vitamin C does not enter the brain.[2] However, the brain is one of the organs which has the greatest concentrations of vitamin C. It is dehydroascorbate that is transported through the blood-brain barrier via de GLUT1 transporters. DHA is then converted to vitamin C. Administration of dehydroascorbic acid confers protection from neuronal injury following stroke (ischemic stroke).
The lack of increased intracerebral hemorrhage (ICH) after the delayed administration of DHA after ischemia confers two significant advantages to this agent, which could lead to its utility at clinically relevant time points. As a well-tolerated agent, it could be given safely in the field before arrival in the hospital and the definitive diagnosis of stroke. Furthermore, its low risk of increasing ICH when administered after stroke obviates the expenditure of valuable time within the therapeutic window by eliminating the prerequisite radiographic imaging studies. Taken together, a pharmacological strategy to increase cerebral levels of ascorbate in stroke has tremendous potential to represent the timely translation of basic research into a relevant therapy for thromboembolic stroke in humans.[2]
References
- ↑ 1.0 1.1 KC S, Carcamo JM, Golde DW (2005). "Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury". FASEB J. 19 (12): 1657–67. PMID 16195374.
- ↑ 2.0 2.1 Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES Jr. (2001). "Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke". Proceedings of the National Academy of Sciences. 98 (20): 11720–11724. PMID 11573006.
See also
Nualart F, Rivas C, Montecinos V, Godoy A, Guaiquil V, Golde D, Vera J (2003). "Recycling of vitamin C by a bystander effect". J Biol Chem. 278 (12): 10128–33. PMID 12435736.