Uterine cancer pathophysiology: Difference between revisions
(Mahshid) |
|||
| Line 200: | Line 200: | ||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Overview incomplete]] | [[Category:Overview incomplete]] | ||
[[Category:Up-To-Date]] | |||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
[[Category:Gynecology]] | |||
Latest revision as of 17:31, 27 November 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
Uterine cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Uterine cancer pathophysiology On the Web |
|
American Roentgen Ray Society Images of Uterine cancer pathophysiology |
|
Risk calculators and risk factors for Uterine cancer pathophysiology |
Overview
Pathophysiology
|
Endometrial cancer forms when there are errors in normal endometrial cell growth.[2] Usually, when cells grow old or get damaged, they die, and new cells take their place.[2] Cancer starts when new cells form unneeded, and old or damaged cells do not die as they should.[2] The buildup of extra cells often forms a mass of tissue called a growth or tumor.[2] These abnormal cancer cells have many genetic abnormalities that cause them to grow excessively.[3]
In 10–20% of endometrial cancers, mostly Grade 3 (the highest histologic grade), mutations are found in a tumor suppressor gene, commonly p53 or PTEN. In 20% of endometrial hyperplasias and 50% of endometrioid cancers, PTEN suffers a loss-of-function mutation or a null mutation, making it less effective or completely ineffective.[4] Loss of PTEN function leads to up-regulation of the PI3k/Akt/mTOR pathway, which causes cell growth.[5] The p53 pathway can either be suppressed or highly activated in endometrial cancer. When a mutant version of p53 is overexpressed, the cancer tends to be particularly aggressive. P53 mutations and chromosome instability are associated with serous carcinomas, which tend to resemble ovarian and Fallopian carcinomas. Serous carcinomas are thought to develop from endometrial intraepithelial carcinoma.[5]
PTEN and p27 loss of function mutations are associated with a good prognosis, particularly in obese women. The Her2/neu oncogene, which indicates a poor prognosis, is expressed in 20% of endometrioid and serous carcinomas. CTNNB1 (beta-catenin; a transcription gene) mutations are found in 14–44% of endometrial cancers and may indicate a good prognosis, but the data is unclear. Beta-catenin mutations are commonly found in endometrial cancers with squamous cells.[5] FGFR2 mutations are found in approximately 10% of endometrial cancers, and their prognostic significance is unclear.[4] SPOP is another tumor suppressor gene found to be mutated in some cases of endometrial cancer: 9% of clear cell endometrial carcinomas and 8% of serous endometrial carcinomas have mutations in this gene.[6]
Type I and Type II cancers (explained below) tend to have different mutations involved. ARID1A, which often carries a point mutation in Type I endometrial cancer, is also mutated in 26% of clear cell carcinomas of the endometrium, and 18% of serous carcinomas. Epigenetic silencing and point mutations of several genes are commonly found in Type I endometrial cancer.[7] Mutations in tumor suppressor genes are common in Type II endometrial cancer. PIK3CA is commonly mutated in both Type I and Type II cancers. In women with Lynch syndrome-associated endometrial cancer, microsatellite instability is common.[5]
Development of an endometrial hyperplasia (overgrowth of endometrial cells) is a significant risk factor because hyperplasias can and often do develop into adenocarcinoma, though cancer can develop without the presence of a hyperplasia. Within ten years, 8–30% of atypical endometrial hyperplasias develop into cancer, whereas 1–3% of non-atypical hyperplasias do so.[8] An atypical hyperplasia is one with visible abnormalities in the nuclei. Pre-cancerous endometrial hyperplasias are also referred to as endometrial intraepithelial neoplasia.[9] Mutations in the KRAS gene can cause endometrial hyperplasia and therefore Type I endometrial cancer. Endometrial hyperplasia typically occurs after the age of 40. Endometrial glandular dysplasia occurs with an overexpression of p53, and develops into a serous carcinoma.[10]
Types
- Histological types include[11]
- Type I
- Endometrioid carcinoma of the endometrium: commonest histological type: ~85%
- Type II
- Papillary serous carcinoma of the endometrium: 5-10% 12
- Clear cell carcinoma of the endometrium: 1-5.5% 11
- Adenosquamous carcinoma of the endometrium: ~2%
- Adenocarcinoma of the endometrium with squamous differentiation: 0.25-0.50% 10
- Undifferentiated carcinoma of the endometrium
- Small cell undifferentied carcinoma of the endometrium
Carcinoma
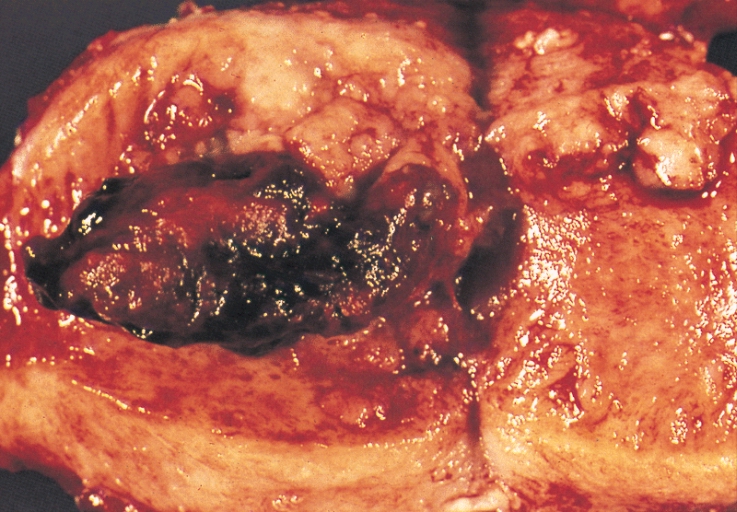
The vast majority of endometrial cancers are carcinomas (usually adenocarcinomas), meaning that they originate from the single layer of epithelial cells that line the endometrium and form the endometrial glands. There are many microscopic subtypes of endometrial carcinoma, but they are broadly organized into two categories, Type I and Type II, based on clinical features and pathogenesis. The two subtypes are genetically distinct. Type I endometrial carcinomas occur most commonly before and around the time of menopause. Type I endometrial cancers are often low-grade, minimally invasive into the underlying uterine wall (myometrium), estrogen-dependent, and have a good outcome with treatment.Type I carcinomas represent 75–90% of endometrial cancer. Type II endometrial carcinomas usually occur in older, post-menopausal people, in the United States are more common in black women, and are not associated with increased exposure to estrogen or a history of endometrial hyperplasia. Type II endometrial cancers are often high-grade, with deep invasion into the underlying uterine wall (myometrium), are of the uterine papillary serous carcinoma|serous or uterine clear cell carcinoma|clear cell type, and carry a poorer prognosis. They can appear to be epithelial ovarian cancer on evaluation of symptoms.[12] They tend to present later than Type I tumors and are more aggressive, with a greater risk of relapse and/or metastasis.[10]
Endometrioid adenocarcinoma
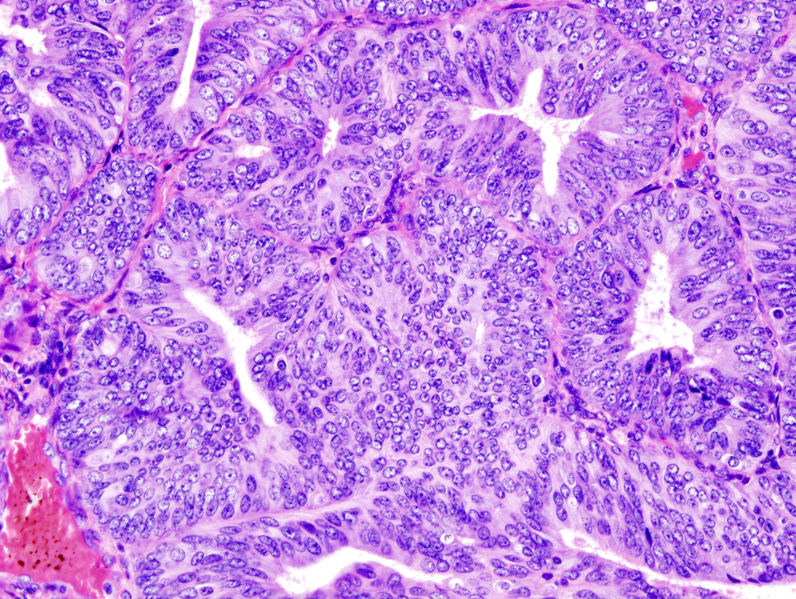
In endometrioid adenocarcinoma, the cancer cells grow in patterns reminiscent of normal endometrium, with many new glands formed from columnar epithelium with some nuclear atypia|abnormal nuclei. Low-grade endometrioid adenocarcinomas have well differentiated cells, have not invaded the myometrium, and are seen alongside endometrial hyperplasia. The tumor's glands form very close together, without the stromal tissue that normally separates them. Higher-grade endometrioid adenocarcinomas have less well-differentiated cells, have more solid sheets of tumor cells no longer organized into glands, and are associated with an atrophied endometrium. There are several subtypes of endometrioid adenocarcinoma with similar prognoses, including villoglandular, secretory, and ciliated cell variants. There is also a subtype characterized by squamous differentiation. Some endometrioid adenocarcinomas have foci of mucinous carcinoma.[13]
The genetic mutations most commonly associated with endometrioid adenocarcinoma are in the genes PTEN, a tumor suppressor; PIK3CA, a kinase; KRAS, a GTPase that functions in signal transduction; and CTNNB1, involved in adhesion and cell signaling. The CTNNB1 (beta-catenin) gene is most commonly mutated in the squamous subtype of endometrioid adenocarcinoma.[14]
Serous carcinoma
Serous carcinoma is a Type II endometrial tumor that makes up 5–10% of diagnosed endometrial cancer and is common in postmenopausal women with atrophied endometrium and black women. Serous endometrial carcinoma is aggressive and often invades the myometrium and metastasizes within the peritoneum (seen as omental cake|omental caking) or the lymphatic system. Histologically, it appears with many atypical nuclei, papillary structures, and, in contrast to endometrioid adenocarcinomas, rounded cells instead of columnar cells. Roughly 30% of endometrial serous carcinomas also have psammoma bodies. Serous carcinomas spread differently than most other endometrial cancers; they can spread outside the uterus without invading the myometrium.The genetic mutations seen in serous carcinoma are chromosomal instability and mutations in TP53, an important tumor suppressor gene.[15]
Clear cell carcinoma
Clear cell carcinoma is a Type II endometrial tumor that makes up less than 5% of diagnosed endometrial cancer. Like serous cell carcinoma, it is usually aggressive and carries a poor prognosis. Histologically, it is characterized by the features common to all clear cells: the eponymous clear cytoplasm when H&E stained and visible, distinct cell membranes.The p53 cell signaling system is not active in endometrial clear cell carcinoma.[16] This form of endometrial cancer is more common in postmenopausal women.
Mucinous carcinoma
Mucinous carcinomas are a rare form of endometrial cancer, making up less than 1–2% of all diagnosed endometrial cancer. Mucinous endometrial carcinomas are most often stage I and grade I, giving them a good prognosis. They typically have well-differentiated columnar cells organized into glands with the characteristic mucin in the cytoplasm. Mucinous carcinomas must be differentiated from cervical adenocarcinoma.
Mixed or undifferentiated carcinoma
Mixed carcinomas are those that have both Type I and Type II cells, with one making up at least 10% of the tumor.These include the malignant mixed Müllerian tumor, which derives from endometrial epithelium and has a poor prognosis.[17].Undifferentiated endometrial carcinomas make up less than 1–2% of diagnosed endometrial cancers. They have a worse prognosis than grade III tumors. Histologically, these tumors show sheets of identical epithelial cells with no identifiable pattern.
Other carcinomas
Non-metastatic squamous cell carcinoma and transitional cell carcinoma are very rare in the endometrium. Squamous cell carcinoma of the endometrium has a poor prognosis. It has been reported fewer than 100 times in the medical literature since its characterization in 1892. For primary squamous cell carcinoma of the endometrium (PSCCE) to be diagnosed, there must be no other primary cancer in the endometrium or cervix and it must not be connected to the cervical epithelium. Because of the rarity of this cancer, there are no guidelines for how it should be treated, nor any typical treatment. The common genetic causes remain uncharacterized.[18] Primary transitional cell carcinomas of the endometrium are even more rare; 16 cases had been reported as of 2008[update]. Its pathophysiology and treatments have not been characterized.[19] Histologically, TCCE resembles endometrioid carcinoma and is distinct from other transitional cell carcinomas.[20]
Sarcoma
In contrast to endometrial carcinomas, the uncommon endometrial stromal sarcomas are cancers that originate in the non-glandular connective tissue of the endometrium. They are generally non-aggressive and, if they recur, can take decades. Metastases to the lungs and pelvic or peritoneal cavities are the most frequent. They typically have estrogen and/or progesterone receptors.[21] The prognosis for low-grade endometrial stromal sarcoma is good, with 60–90% five-year survival. High-grade undifferentiated endometrial sarcoma (HGUS) has a worse prognosis, with high rates of recurrence and 25% five-year survival.[22] HGUS prognosis is dictated by whether or not the cancer has invaded the arteries and veins. Without vascular invasion, the five-year survival is 83%; it drops to 17% when vascular invasion is observed. Stage I ESS has the best prognosis, with five-year survival of 98% and ten-year survival of 89%. ESS makes up 0.2% of uterine cancers.[23]
Metastasis
Endometrial cancer frequently metastasizes to the ovaries and Fallopian tubes when the cancer is located in the upper part of the uterus, and the cervix when the cancer is in the lower part of the uterus. The cancer usually first spreads into the myometrium and the serosa, then into other reproductive and pelvic structures. When the lymphatic system is involved, the pelvic and para-aortic nodes are usually first to become involved, but in no specific pattern, unlike cervical cancer. More distant metastases are spread by the blood and often occur in the lungs, as well as the liver, brain, and bone.[24] Endometrial cancer metastasizes to the lungs 20–25% of the time, more than any other gynecologic cancer.[25]
Histopathology
There is a three-tiered system for histologically classifying endometrial cancers, ranging from cancers with well-differentiated cells (grade I), to very poorly-differentiated cells (grade III).[26] Grade I cancers are the least aggressive and have the best prognosis, while grade III tumors are the most aggressive and likely to recur. Grade II cancers are intermediate between grades I and III in terms of cell differentiation and aggressiveness of disease.
The histopathology of endometrial cancers is highly diverse. The most common finding is a well-differentiated endometrioid adenocarcinoma,[17] which is composed of numerous, small, crowded glands with varying degrees of nuclear atypia, mitotic activity, and stratification. This often appears on a background of endometrial hyperplasia. Frank adenocarcinoma may be distinguished from atypical hyperplasia by the finding of clear stromal invasion, or "back-to-back" glands which represent nondestructive replacement of the endometrial stroma by the cancer. With progression of the disease, the myometrium is infiltrated.[27]
References
- ↑ International Agency for Research on Cancer (2014). World Cancer Report 2014. World Health Organization. Chapter 5.12. ISBN 978-92-832-0429-9.
- ↑ 2.0 2.1 2.2 2.3 Kong A, Johnson N, Kitchener HC, Lawrie TA (2012). "Adjuvant radiotherapy for stage I endometrial cancer". Cochrane Database Syst Rev. 4: CD003916. doi:10.1002/14651858.CD003916.pub4. PMC 4164955. PMID 22513918.
- ↑ What You Need To Know: Endometrial Cancer".NCI. National Cancer Institute. Retrieved 6 August 2014.
- ↑ 4.0 4.1 Thaker, PH; Sood, AK. "Molecular Oncology in Gynecologic Cancer". In Lentz, GM; Lobo, RA; Gershenson, DM; Katz, VL. Comprehensive Gynecology (6th ed.). Mosby. ISBN 978-0-323-06986-1.
- ↑ 5.0 5.1 5.2 5.3 Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C; et al. (2013). "Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Ann Oncol. 24 Suppl 6: vi33–8. doi:10.1093/annonc/mdt353. PMID 24078661.
- ↑ Mani, RS (September 2014). "The emerging role of speckle-type POZ protein (SPOP) in cancer development". Drug Discovery Today. 19 (9): 1498–1502. doi:10.1016/j.drudis.2014.07.009. PMID 25058385.
A recent exome-sequencing study revealed that 8% of serious endometrial cancers and 9% of clear cell endometrial cancers have SPOP mutations
- ↑ International Agency for Research on Cancer (2014). World Cancer Report 2014. World Health Organization. Chapter 5.12. ISBN 978-92-832-0429-9.
- ↑ Luo, L; Luo, B; Zheng, Y; Zhang, H; Li, J; Sidell, N (5 June 2013). "Levonorgestrel-releasing intrauterine system for atypical endometrial hyperplasia". The Cochrane database of systematic reviews. 6: CD009458. doi:10.1002/14651858.CD009458.pub2. PMID 23737032.
- ↑ Hoffman, BL; Schorge, JO; Schaffer, JI; Halvorson, LM; Bradshaw, KD; Cunningham, FG, eds. (2012). "Endometrial Cancer". Williams Gynecology (2nd ed.). McGraw-Hill. p. 820. ISBN 978-0-07-171672-7.
- ↑ 10.0 10.1 Saso, S; Chatterjee, J; Georgiou, E; Ditri, AM; Smith, JR; Ghaem-Maghami, S (2011). "Endometrial cancer". BMJ. 343: d3954–d3954. doi:10.1136/bmj.d3954. PMID 21734165.
- ↑ Amir Rezaee◉ and Dr Yuranga Weerakkody◉ et al "endometrial cancer" Check
|url=value (help). - ↑ Hoffman, BL; Schorge, JO; Schaffer, JI; Halvorson, LM; Bradshaw, KD; Cunningham, FG, eds. (2012). "Endometrial Cancer". Williams Gynecology (2nd ed.). McGraw-Hill. p. 826. ISBN 978-0-07-171672-7.
- ↑ Hoffman, BL; Schorge, JO; Schaffer, JI; Halvorson, LM; Bradshaw, KD; Cunningham, FG, eds. (2012). "Endometrial Cancer". Williams Gynecology (2nd ed.). McGraw-Hill. p. 827. ISBN 978-0-07-171672-7.
- ↑ Colombo, N; Preti, E; Landoni, F; Carinelli, S; Colombo, A; Marini, C; Sessa, C (2011). "Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of Oncology. 22 (Supplement 6): vi35–vi39. doi:10.1093/annonc/mdr374. PMID 21908501.
- ↑ Johnson N, Bryant A, Miles T, Hogberg T, Cornes P (2011). "Adjuvant chemotherapy for endometrial cancer after hysterectomy". Cochrane Database Syst Rev (10): CD003175. doi:10.1002/14651858.CD003175.pub2. PMC 4164379. PMID 21975736.
- ↑ Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S (2011). "Endometrial cancer". BMJ. 343: d3954. doi:10.1136/bmj.d3954. PMID 21734165.
- ↑ 17.0 17.1 Johnson, N; Bryant, A; Miles, T; Hogberg, T; Cornes, P (5 October 2011). "Adjuvant chemotherapy for endometrial cancer after hysterectomy". The Cochrane database of systematic reviews (10): CD003175. doi:10.1002/14651858.CD003175.pub2. PMID 21975736.
- ↑ Goodrich, S; Kebria-Moslemi, M; Broshears, J; Sutton, GP; Rose, P (September 2013). "Primary squamous cell carcinoma of the endometrium: two cases and a review of the literature". Diagnostic Cytopathology. 41 (9): 817–20. doi:10.1002/dc.22814. PMID 22241749.
- ↑ Mariño-Enríquez, A; González-Rocha, T; Burgos, E (November 2008). et al. "Transitional cell carcinoma of the endometrium and endometrial carcinoma with transitional cell differentiation: a clinicopathologic study of 5 cases and review of the literature". Human Pathology. 39 (11): 1606–13. doi:10.1016/j.humpath.2008.03.005. PMID 18620731.
- ↑ Ahluwalia, M; Light, AM; Surampudi, K; Finn, CB (October 2006). "Transitional cell carcinoma of the endometrium: a case report and review of the literature". International Journal of Gynecological Pathology. 25 (4): 378–82. doi:10.1097/01.pgp.0000215296.53361.4b. PMID 16990716.
- ↑ Sylvestre, VT; Dunton, CJ (April 2010). "Treatment of recurrent endometrial stromal sarcoma with letrozole: a case report and literature review". Hormones and Cancer. 1 (2): 112–5. doi:10.1007/s12672-010-0007-9. PMID 21761354.
- ↑ Hensley ML (2012). "Uterine sarcomas: histology and its implications on therapy". American Society of Clinical Oncology educational book: 356–61. doi:10.14694/EdBook_AM.2012.32.356. PMID 24451763.
- ↑ D'Angelo, E; Prat, J (January 2010). "Uterine sarcomas: a review". Gynecologic Oncology. 116 (1): 131–9. doi:10.1016/j.ygyno.2009.09.023. PMID 19853898.
- ↑ Hoffman, BL; Schorge, JO; Schaffer, JI; Halvorson, LM; Bradshaw, KD; Cunningham, FG, eds. (2012). "Endometrial Cancer". Williams Gynecology (2nd ed.). McGraw-Hill. p. 828. ISBN 978-0-07-171672-7.
- ↑ Kurra V, Krajewski KM, Jagannathan J, Giardino A, Berlin S, Ramaiya N (2013). "Typical and atypical metastatic sites of recurrent endometrial carcinoma". Cancer Imaging. 13: 113–22. doi:10.1102/1470-7330.2013.0011. PMC 3613792. PMID 23545091.
- ↑ Vale CL, Tierney J, Bull SJ, Symonds PR (2012). "Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma". Cochrane Database Syst Rev. 8: CD003915. doi:10.1002/14651858.CD003915.pub4. PMID 22895938.
- ↑ Weidner, N; Coté, R; Suster, S; Weiss, L, eds. (2002). Modern Surgical Pathology (2 Volume Set). WB Saunders. ISBN 978-0-7216-7253-3.
- CS1 maint: Multiple names: authors list
- CS1 maint: PMC format
- CS1 maint: display-editors
- CS1 maint: Explicit use of et al.
- Pages with URL errors
- Articles containing potentially dated statements from 2008
- Articles with invalid date parameter in template
- All articles containing potentially dated statements
- Gynecology
- Disease
- Overview incomplete
- Up-To-Date
- Oncology
- Medicine