Rabies causes: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
{{CMG}} | {{CMG}} | ||
{{about|the virus|the disease|Rabies|other uses|Rabies (disambiguation)}} | |||
{{Taxobox | |||
| name = Rabies | |||
| image = Rabies Virus EM PHIL 1876.JPG | |||
| image width = 240px | |||
| image_caption = [[Transmission electron microscopy|TEM]] [[micrograph]] with numerous rabies [[virion]]s (small dark-grey rod-like particles) and Negri bodies (the larger [[pathognomonic]] cellular inclusions of rabies infection) | |||
| virus_group = v | |||
| ordo = ''[[Mononegavirales]]'' | |||
| familia = ''[[Rhabdoviridae]]'' | |||
| genus = ''[[Lyssavirus]]'' | |||
| species = '''''Rabies virus''''' | |||
}} | |||
The | The '''rabies virus''' is a [[neurotropic virus]] that causes [[rabies]] in humans and animals. [[Rabies transmission]] can occur through the saliva of animals and less commonly through contact with human saliva. | ||
The rabies virus has a cylindrical morphology and is the [[type species]] of the ''[[Lyssavirus]]'' [[genus]] of the ''[[Rhabdoviridae]]'' family. These viruses are [[Viral envelope|enveloped]] and have a single stranded [[RNA]] genome with [[negative-sense]]. The genetic information is packaged as a [[ribonucleoprotein]] complex in which RNA is tightly bound by the viral nucleoprotein. The RNA genome of the virus encodes five genes whose order is highly conserved. These genes code for nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and the viral RNA polymerase (L).<ref name="pmid15885837">{{cite journal |author=Finke S, Conzelmann KK |title=Replication strategies of rabies virus |journal=Virus Res. |volume=111 |issue=2 |pages= 120–131 |date=August 2005 |pmid=15885837 |doi=10.1016/j.virusres.2005.04.004 |url=}}</ref> The complete genome sequences range from 11,615 to 11,966 nt in length.<ref>{{cite web | url=http://www.ncbi.nlm.nih.gov/nuccore/?term=rabies+complete+genome |title=Rabies complete genome | accessdate=29 May 2013 |publisher=[[National Center for Biotechnology Information|NCBI]] Nucleotide Database}}</ref> | |||
All transcription and replication events take place in the cytoplasm inside a specialized “virus factory”, the ''[[Negri bodies|Negri body]]'' (named after [[Adelchi Negri]]<ref>{{WhoNamedIt|synd|2491}}</ref>). These are 2–10 [[micrometre|µm]] in diameter and are typical for a rabies infection and thus have been used as [[pathognomonic|definite histological proof of such infection]].<ref name="pmid17938861">{{cite journal |author=Albertini AA, Schoehn G, Weissenhorn W, Ruigrok RW |title=Structural aspects of rabies virus replication |journal=Cell. Mol. Life Sci. |volume=65 |issue=2 |pages= 282–294 |date=January 2008 |pmid=17938861 |doi=10.1007/s00018-007-7298-1 |url=}}</ref> | |||
==Structure== | |||
Lyssaviruses have [[helical]] symmetry, so their infectious particles are approximately cylindrical in shape. They are characterized by an extremely broad host spectrum ranging from plants to insects and mammals; human-infecting viruses more commonly have cubic symmetry and take shapes approximating [[regular polyhedron|regular polyhedra]]. | |||
The rabies virus has a bullet like shape with a length of about 180 [[nanometer|nm]] and a cross-sectional diameter of about 75 nm. One end is rounded or conical and the other end is planar or concave. The [[lipoprotein]] envelope carries knob-like spikes composed of [[Glycoprotein]] G. Spikes do not cover the planar end of the virion (virus particle). Beneath the envelope is the membrane or matrix (M) protein layer which may be [[invaginated]] at the planar end. The core of the virion consists of helically arranged [[ribonucleoprotein]]. | |||
==Life cycle== | |||
{{Viral life cycle}} | |||
After receptor binding, rabies virus enters its host cells through the [[Endosome|endosomal]] transport pathway. Inside the endosome, the low [[pH]] value induces the membrane fusion process, thus enabling the viral genome to reach the [[cytosol]]. Both processes, receptor binding and membrane fusion, are catalyzed by the glycoprotein G which plays a critical role in pathogenesis (mutant virus without G proteins cannot propagate).<ref name="pmid15885837"/> | |||
The next step after entry is the [[Transcription (genetics)|transcription]] of the viral genome by the P-L polymerase (P is an essential cofactor for the L polymerase) in order to make new viral protein. The viral polymerase can only recognize [[ribonucleoprotein]] and cannot use free RNA as template. Transcription is regulated by [[Cis-regulatory element|''cis''-acting sequences]] on the virus genome and by protein M which is not only essential for virus budding but also regulates the fraction of mRNA production to replication. Later in infection, the activity of the polymerase switches to replication in order to produce full-length positive-strand RNA copies. These complementary RNAs are used as templates to make new negative-strand RNA genomes. They are packaged together with protein N to form [[ribonucleoprotein]] which then can form new viruses.<ref name="pmid17938861"/> | |||
==Infection== | |||
In September 1931, [[Joseph Lennox Pawan]] of [[Trinidad]] in the [[West Indies]], a Government Bacteriologist, found [[Negri bodies]] in the brain of a bat with unusual habits. In 1932, Pawan first discovered that infected [[vampire bats]] could transmit rabies to humans and other animals.<ref>{{cite journal |last=Pawan |first=J. L. |year=1936 |title=Transmission of the Paralytic Rabies in Trinidad of the Vampire Bat: ''Desmodus rotundus murinus'' Wagner, 1840 |journal=Annals of Tropical Medicine and Parasitology |issn=0003-4983 |volume=30 |issue= |pages=137–156 }}</ref><ref>{{cite journal |last=Pawan |first=J. L. |title=Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection |journal=Ann Trop Med Parasitol |year=1936 |volume=30 |issue= |pages=101–129 |issn=0003-4983 }}</ref> For a brief history of some of the controversies surrounding the early discoveries relating to rabies in Trinidad, see the brief history by James Waterman.<ref>{{cite journal |last=Waterman |first=James A. |title=The History of the Outbreak of Paralytic Rabies in Trinidad Transmitted by Bats to Human beings and Lower animals from 1925 |journal=Caribbean Medical Journal |volume=26 |issue=1–4 |year=1965 |pages=164–169 |issn=0374-7042 }}</ref> | |||
From the wound of entry, the rabies virus travels quickly along the neural pathways of the [[peripheral nervous system]]. The [[Axoplasmic transport|retrograde axonal transport]] of the rabies virus to the CNS ([[Central Nervous System]]) is the key step of pathogenesis during natural infection. The exact molecular mechanism of this transport is unknown although binding of the P protein from rabies virus to the [[dynein]] light chain protein [[DYNLL1]] has been shown.<ref name="pmid11024151">{{cite journal |author=Raux H, Flamand A, Blondel D |title=Interaction of the rabies virus P protein with the LC8 dynein light chain |journal=J. Virol. |volume=74 |issue=21 |pages= 10212–10216 |date=November 2000 |pmid=11024151 |pmc=102061 |doi= 10.1128/JVI.74.21.10212-10216.2000|url=http://jvi.asm.org/cgi/pmidlookup?view=long&pmid=11024151}}</ref> P also acts as an [[interferon]] antagonist, thus decreasing the [[immune]] response of the host. | |||
From the CNS, the virus further spreads to other organs. The salivary glands located in the tissues of the mouth and cheeks receive high concentrations of the virus, thus allowing it to be further transmitted due to projectile salivation. Fatality can occur from two days to five years from the time of initial infection.<ref>{{cite web | url=http://www.unbc.ca/nlui/wildlife_diseases_bc/rabies.htm | title=Rabies | accessdate=2008-10-10 | author= | last= | first= | authorlink= | coauthors= | date= | year= | work= | publisher=University of Northern British Columbia | location= | pages= | language= | doi= | archiveurl= | archivedate= | quote=}}</ref> This however depends largely on the species of animal acting as a [[Natural reservoir|reservoir]]. Most infected mammals die within weeks, while strains of a species such as the African [[Yellow Mongoose]] (''Cynictis penicillata'') might survive an infection asymptomatically for years.<ref name="pmid7777324">{{cite journal |author=Taylor PJ |title=A systematic and population genetic approach to the rabies problem in the yellow mongoose (Cynictis penicillata) |journal=Onderstepoort J. Vet. Res. |volume=60 |issue=4 |pages=379–87 |date=December 1993 |pmid=7777324 |doi= |url=}}</ref> | |||
==Antigenicity== | |||
Upon viral entry into the body and also after [[Rabies vaccine|vaccination]], the body produces virus neutralizing antibodies which bind and inactivate the virus. Specific regions of the G protein have been shown to be most antigenic in leading to the production of virus neutralizing antibodies. These antigenic sites, or epitopes, are categorized into regions I-IV and minor site a. Previous work has demonstrated that antigenic sites II and III are most commonly targeted by natural neutralizing antibodies.<ref>{{cite journal |author=Benmansour A |title=Antigenicity of rabies virus glycoprotein |journal=Journal of Virology |volume=65 |issue=8 |pages=4198–4203 |year=1991|pmid=1712859|doi= |url= |pmc=248855}}</ref> Additionally, a monoclonal antibody with neutralizing functionality has been demonstrated to target antigenic site I.<ref>{{Cite journal | last1 = Marissen | first1 = WE. | last2 = Kramer | first2 = RA. | last3 = Rice | first3 = A. | last4 = Weldon | first4 = WC. | last5 = Niezgoda | first5 = M. | last6 = Faber | first6 = M. | last7 = Slootstra | first7 = JW. | last8 = Meloen | first8 = RH. | last9 = Clijsters-van der Horst | first9 = M. | last10 = Visser | first10 = T. J. | last11 = Jongeneelen | first11 = M. | last12 = Thijsse | first12 = S. | last13 = Throsby | first13 = M. | last14 = De Kruif | first14 = J. | last15 = Rupprecht | first15 = C. E. | last16 = Dietzschold | first16 = B. | last17 = Goudsmit | first17 = J. | last18 = Bakker | first18 = A. B. H. | title = Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis | journal = J Virol | volume = 79 | issue = 8 | pages = 4672–8 |date=Apr 2005 | doi = 10.1128/JVI.79.8.4672-4678.2005 | pmid = 15795253 | display-authors = 8 | pmc = 1069557 }}</ref> Other proteins, such as the nucleoprotein, have been shown to be unable to elicit production of virus neutralizing antibodies.<ref name="Wiktor-1973">{{Cite journal | last1 = Wiktor | first1 = TJ. | last2 = György | first2 = E. | last3 = Schlumberger | first3 = D. | last4 = Sokol | first4 = F. | last5 = Koprowski | first5 = H. | title = Antigenic properties of rabies virus components | journal = J Immunol | volume = 110 | issue = 1 | pages = 269–76 |date=Jan 1973 | doi = | pmid = 4568184 }}</ref> The epitopes which bind neutralizing antibodies are both linear and conformational.<ref name="Bakker-2005">{{Cite journal | last1 = Bakker | first1 = AB. | last2 = Marissen | first2 = WE. | last3 = Kramer | first3 = RA. | last4 = Rice | first4 = AB. | last5 = Weldon | first5 = WC. | last6 = Niezgoda | first6 = M. | last7 = Hanlon | first7 = CA. | last8 = Thijsse | first8 = S. | last9 = Backus | first9 = HH. | last10 = De Kruif | first10 = J. | last11 = Dietzschold | first11 = B. | last12 = Rupprecht | first12 = C. E. | last13 = Goudsmit | first13 = J. | title = Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants | journal = J Virol | volume = 79 | issue = 14 | pages = 9062–8 |date=Jul 2005 | doi = 10.1128/JVI.79.14.9062-9068.2005 | pmid = 15994800 | display-authors = 8 | pmc = 1168753 }}</ref> | |||
===Transmission=== | ===Transmission=== | ||
| Line 28: | Line 56: | ||
Transmission between humans is extremely rare. A few cases have been recorded through [[organ transplant|transplant surgery]].<ref>{{cite journal | author = Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR | title = Transmission of rabies virus from an organ donor to four transplant recipients | journal = [[New England Journal of Medicine|N Engl J Med]] | volume = 352 | issue = 11 | pages = 1103–11 | date = March 2005 | pmid = 15784663 | doi = 10.1056/NEJMoa043018 | url = http://www.nejm.org/doi/pdf/10.1056/NEJMoa043018 | format = PDF }}</ref> After a typical human infection by bite, the virus enters the [[peripheral nervous system]]. It then travels along the [[Afferent nerve fiber|afferent nerve]]s toward the [[central nervous system]].<ref>{{cite book |author=Jackson, Alan C., Wunner, William H.|title=Rabies|url=http://books.google.com/books?id=p8rMezRaD4oC&pg=PA290 |year=2002|publisher=Academic Press|isbn=978-0-12-379077-4|page=290}}</ref> During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. When the virus reaches the [[brain]], it rapidly causes [[encephalitis]], the prodromal phase, and is the beginning of the symptoms. Once the patient becomes symptomatic, treatment is almost never effective and mortality is over 99%. Rabies may also inflame the [[spinal cord]], producing transverse [[myelitis]].<ref>Joanne Lynn, M.D. (October 1997) [http://www.myelitis.org/tm.htm ''Transverse Myelitis: Symptoms, Causes and Diagnosis''] The Transverse Myelitis Association</ref><ref>{{cite book|author1=Larry Ernest Davis|author2=Molly K. King|author3=Jessica L. Schultz|title=Fundamentals of neurologic disease|url=http://books.google.com/books?id=moRp2jWZp0QC&pg=PA73 |date=15 June 2005|publisher=Demos Medical Publishing|isbn=978-1-888799-84-2|page=73}}</ref> | Transmission between humans is extremely rare. A few cases have been recorded through [[organ transplant|transplant surgery]].<ref>{{cite journal | author = Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR | title = Transmission of rabies virus from an organ donor to four transplant recipients | journal = [[New England Journal of Medicine|N Engl J Med]] | volume = 352 | issue = 11 | pages = 1103–11 | date = March 2005 | pmid = 15784663 | doi = 10.1056/NEJMoa043018 | url = http://www.nejm.org/doi/pdf/10.1056/NEJMoa043018 | format = PDF }}</ref> After a typical human infection by bite, the virus enters the [[peripheral nervous system]]. It then travels along the [[Afferent nerve fiber|afferent nerve]]s toward the [[central nervous system]].<ref>{{cite book |author=Jackson, Alan C., Wunner, William H.|title=Rabies|url=http://books.google.com/books?id=p8rMezRaD4oC&pg=PA290 |year=2002|publisher=Academic Press|isbn=978-0-12-379077-4|page=290}}</ref> During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. When the virus reaches the [[brain]], it rapidly causes [[encephalitis]], the prodromal phase, and is the beginning of the symptoms. Once the patient becomes symptomatic, treatment is almost never effective and mortality is over 99%. Rabies may also inflame the [[spinal cord]], producing transverse [[myelitis]].<ref>Joanne Lynn, M.D. (October 1997) [http://www.myelitis.org/tm.htm ''Transverse Myelitis: Symptoms, Causes and Diagnosis''] The Transverse Myelitis Association</ref><ref>{{cite book|author1=Larry Ernest Davis|author2=Molly K. King|author3=Jessica L. Schultz|title=Fundamentals of neurologic disease|url=http://books.google.com/books?id=moRp2jWZp0QC&pg=PA73 |date=15 June 2005|publisher=Demos Medical Publishing|isbn=978-1-888799-84-2|page=73}}</ref> | ||
==Evolution== | |||
All extant rabies viruses appear to have evolved within the last 1500 years.<ref name=Nadin-Davis2011>{{cite journal |last=Nadin-Davis |first=S. A. |last2=Real |first2=L. A. |year=2011 |title=Molecular phylogenetics of the lyssaviruses--insights from a coalescent approach |journal=Adv Virus Res |volume=79 |issue= |pages=203–238 |pmid=21601049 |doi=10.1016/B978-0-12-387040-7.00011-1 |series=Advances in Virus Research |isbn=9780123870407 }}</ref> There are seven genotypes of rabies virus. In Eurasia cases are due to three of these—genotype 1 (classical rabies) and to a lesser extent genotypes 5 and 6 (European bat lyssaviruses type-1 and -2).<ref name=McElhinney2008>{{cite journal |last=McElhinney |first=L. M. |last2=Marston |first2=D. A. |last3=Stankov |first3=S |last4=Tu |first4=C. |last5=Black |first5=C. |last6=Johnson |first6=N. |last7=Jiang |first7=Y. |last8=Tordo |first8=N. |last9=Müller |first9=T. |last10=Fooks |first10=A. R. |year=2008 |title=Molecular epidemiology of lyssaviruses in Eurasia |journal=Dev Biol (Basel) |volume=131 |issue= |pages=125–131 |doi= |pmid=18634471 }}</ref> Genotype 1 evolved in Europe in the 17th century and spread to Asia, Africa and the Americas as a result of European exploration and colonization. | |||
Bat rabies in North America appears to have been present since 1281 [[Common Era|CE]] (95% [[confidence interval]]: 906–1577 [[Common Era|CE]]).<ref name=Kuzmina2013>{{cite journal |last=Kuzmina |first=N. A. |last2=Kuzmin |first2=I. V. |last3=Ellison |first3=J. A. |last4=Taylor |first4=S. T. |last5=Bergman |first5=D. L. |last6=Dew |first6=B. |last7=Rupprecht |first7=C. E. |year=2013 |title=A reassessment of the evolutionary timescale of bat rabies viruses based upon glycoprotein gene sequences |journal=Virus Genes |volume=Forthcoming |issue= 2|pages= 305|doi=10.1007/s11262-013-0952-9 }}</ref> | |||
==Application== | |||
Rabies virus is used in research for [[viral neuronal tracing]] to establish synaptic connections and directionality of synaptic transmission. <ref name = Ginger>Ginger, M., Haberl M., Conzelmann K.-K., Schwarz M. and Frick A. (2013). Revealing the secrets of neuronal circuits with recombinant rabies virus technology. Front. Neural Circuits. {{doi|10.3389/fncir.2013.00002}}</ref> | |||
==See also== | |||
* [[Cryptic bat rabies]] | |||
* [[Rabies vaccine]] | |||
* [[Duck embryo vaccine]] | |||
* [[Arctic rabies virus]] | |||
* [[Bat-borne virus]] | |||
==References== | ==References== | ||
{{Reflist|30em}} | |||
{{reflist|2}} | {{reflist|2}} | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
==External links== | |||
* [http://www.viprbrc.org/brc/home.do?decorator=rhabdo Virus Pathogen Database and Analysis Resource (ViPR): Rhabdoviridae] | |||
{{Viral diseases}} | |||
[[Category:Disease]] | [[Category:Disease]] | ||
Revision as of 19:58, 7 August 2015
|
Rabies Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rabies causes On the Web |
|
American Roentgen Ray Society Images of Rabies causes |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
| style="background:#Template:Taxobox colour;"|Rabies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
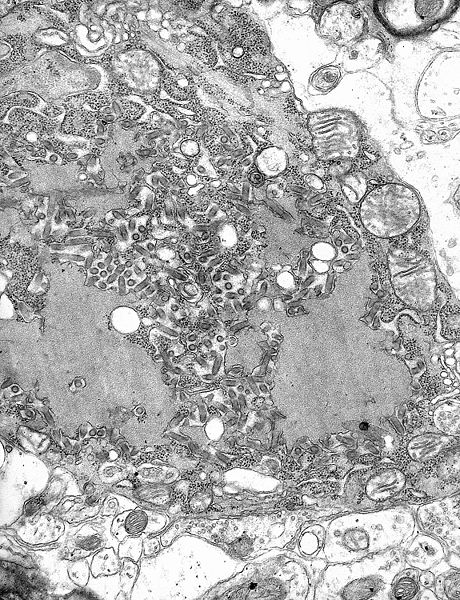 TEM micrograph with numerous rabies virions (small dark-grey rod-like particles) and Negri bodies (the larger pathognomonic cellular inclusions of rabies infection)
| ||||||||||
| style="background:#Template:Taxobox colour;" | Virus classification | ||||||||||
|
The rabies virus is a neurotropic virus that causes rabies in humans and animals. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva.
The rabies virus has a cylindrical morphology and is the type species of the Lyssavirus genus of the Rhabdoviridae family. These viruses are enveloped and have a single stranded RNA genome with negative-sense. The genetic information is packaged as a ribonucleoprotein complex in which RNA is tightly bound by the viral nucleoprotein. The RNA genome of the virus encodes five genes whose order is highly conserved. These genes code for nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and the viral RNA polymerase (L).[1] The complete genome sequences range from 11,615 to 11,966 nt in length.[2]
All transcription and replication events take place in the cytoplasm inside a specialized “virus factory”, the Negri body (named after Adelchi Negri[3]). These are 2–10 µm in diameter and are typical for a rabies infection and thus have been used as definite histological proof of such infection.[4]
Structure
Lyssaviruses have helical symmetry, so their infectious particles are approximately cylindrical in shape. They are characterized by an extremely broad host spectrum ranging from plants to insects and mammals; human-infecting viruses more commonly have cubic symmetry and take shapes approximating regular polyhedra.
The rabies virus has a bullet like shape with a length of about 180 nm and a cross-sectional diameter of about 75 nm. One end is rounded or conical and the other end is planar or concave. The lipoprotein envelope carries knob-like spikes composed of Glycoprotein G. Spikes do not cover the planar end of the virion (virus particle). Beneath the envelope is the membrane or matrix (M) protein layer which may be invaginated at the planar end. The core of the virion consists of helically arranged ribonucleoprotein.
Life cycle
Template:Viral life cycle After receptor binding, rabies virus enters its host cells through the endosomal transport pathway. Inside the endosome, the low pH value induces the membrane fusion process, thus enabling the viral genome to reach the cytosol. Both processes, receptor binding and membrane fusion, are catalyzed by the glycoprotein G which plays a critical role in pathogenesis (mutant virus without G proteins cannot propagate).[1]
The next step after entry is the transcription of the viral genome by the P-L polymerase (P is an essential cofactor for the L polymerase) in order to make new viral protein. The viral polymerase can only recognize ribonucleoprotein and cannot use free RNA as template. Transcription is regulated by cis-acting sequences on the virus genome and by protein M which is not only essential for virus budding but also regulates the fraction of mRNA production to replication. Later in infection, the activity of the polymerase switches to replication in order to produce full-length positive-strand RNA copies. These complementary RNAs are used as templates to make new negative-strand RNA genomes. They are packaged together with protein N to form ribonucleoprotein which then can form new viruses.[4]
Infection
In September 1931, Joseph Lennox Pawan of Trinidad in the West Indies, a Government Bacteriologist, found Negri bodies in the brain of a bat with unusual habits. In 1932, Pawan first discovered that infected vampire bats could transmit rabies to humans and other animals.[5][6] For a brief history of some of the controversies surrounding the early discoveries relating to rabies in Trinidad, see the brief history by James Waterman.[7]
From the wound of entry, the rabies virus travels quickly along the neural pathways of the peripheral nervous system. The retrograde axonal transport of the rabies virus to the CNS (Central Nervous System) is the key step of pathogenesis during natural infection. The exact molecular mechanism of this transport is unknown although binding of the P protein from rabies virus to the dynein light chain protein DYNLL1 has been shown.[8] P also acts as an interferon antagonist, thus decreasing the immune response of the host.
From the CNS, the virus further spreads to other organs. The salivary glands located in the tissues of the mouth and cheeks receive high concentrations of the virus, thus allowing it to be further transmitted due to projectile salivation. Fatality can occur from two days to five years from the time of initial infection.[9] This however depends largely on the species of animal acting as a reservoir. Most infected mammals die within weeks, while strains of a species such as the African Yellow Mongoose (Cynictis penicillata) might survive an infection asymptomatically for years.[10]
Antigenicity
Upon viral entry into the body and also after vaccination, the body produces virus neutralizing antibodies which bind and inactivate the virus. Specific regions of the G protein have been shown to be most antigenic in leading to the production of virus neutralizing antibodies. These antigenic sites, or epitopes, are categorized into regions I-IV and minor site a. Previous work has demonstrated that antigenic sites II and III are most commonly targeted by natural neutralizing antibodies.[11] Additionally, a monoclonal antibody with neutralizing functionality has been demonstrated to target antigenic site I.[12] Other proteins, such as the nucleoprotein, have been shown to be unable to elicit production of virus neutralizing antibodies.[13] The epitopes which bind neutralizing antibodies are both linear and conformational.[14]
Transmission
All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. Birds were first artificially infected with rabies in 1884; however, infected birds are largely if not wholly asymptomatic, and recover.[15] Other bird species have been known to develop rabies antibodies, a sign of infection, after feeding on rabies-infected mammals.[16][17]
The virus has also been adapted to grow in cells of poikilothermic ("cold-blooded") vertebrates.[18][19] Most animals can be infected by the virus and can transmit the disease to humans. Infected bats,[20][21] monkeys, raccoons, foxes, skunks, cattle, wolves, coyotes, dogs, mongooses (normally yellow mongoose)[22] and cats present the greatest risk to humans.
Rabies may also spread through exposure to infected domestic farm animals, groundhogs, weasels, bears, and other wild carnivorans. Small rodents, such as squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, and mice, and lagomorphs such as rabbits and hares, are almost never found to be infected with rabies and are not known to transmit rabies to humans.[23] The Virginia opossum is resistant but not immune to rabies.[24]
The virus is usually present in the nerves and saliva of a symptomatic rabid animal.[25][26] The route of infection is usually, but not always, by a bite. In many cases, the infected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behavior.[27] This is an example of a viral pathogen modifying the behavior of its host to facilitate its transmission to other hosts.
Transmission between humans is extremely rare. A few cases have been recorded through transplant surgery.[28] After a typical human infection by bite, the virus enters the peripheral nervous system. It then travels along the afferent nerves toward the central nervous system.[29] During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. When the virus reaches the brain, it rapidly causes encephalitis, the prodromal phase, and is the beginning of the symptoms. Once the patient becomes symptomatic, treatment is almost never effective and mortality is over 99%. Rabies may also inflame the spinal cord, producing transverse myelitis.[30][31]
Evolution
All extant rabies viruses appear to have evolved within the last 1500 years.[32] There are seven genotypes of rabies virus. In Eurasia cases are due to three of these—genotype 1 (classical rabies) and to a lesser extent genotypes 5 and 6 (European bat lyssaviruses type-1 and -2).[33] Genotype 1 evolved in Europe in the 17th century and spread to Asia, Africa and the Americas as a result of European exploration and colonization.
Bat rabies in North America appears to have been present since 1281 CE (95% confidence interval: 906–1577 CE).[34]
Application
Rabies virus is used in research for viral neuronal tracing to establish synaptic connections and directionality of synaptic transmission. [35]
See also
References
- ↑ 1.0 1.1 Finke S, Conzelmann KK (August 2005). "Replication strategies of rabies virus". Virus Res. 111 (2): 120–131. doi:10.1016/j.virusres.2005.04.004. PMID 15885837.
- ↑ "Rabies complete genome". NCBI Nucleotide Database. Retrieved 29 May 2013.
- ↑ Template:WhoNamedIt
- ↑ 4.0 4.1 Albertini AA, Schoehn G, Weissenhorn W, Ruigrok RW (January 2008). "Structural aspects of rabies virus replication". Cell. Mol. Life Sci. 65 (2): 282–294. doi:10.1007/s00018-007-7298-1. PMID 17938861.
- ↑ Pawan, J. L. (1936). "Transmission of the Paralytic Rabies in Trinidad of the Vampire Bat: Desmodus rotundus murinus Wagner, 1840". Annals of Tropical Medicine and Parasitology. 30: 137–156. ISSN 0003-4983.
- ↑ Pawan, J. L. (1936). "Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection". Ann Trop Med Parasitol. 30: 101–129. ISSN 0003-4983.
- ↑ Waterman, James A. (1965). "The History of the Outbreak of Paralytic Rabies in Trinidad Transmitted by Bats to Human beings and Lower animals from 1925". Caribbean Medical Journal. 26 (1–4): 164–169. ISSN 0374-7042.
- ↑ Raux H, Flamand A, Blondel D (November 2000). "Interaction of the rabies virus P protein with the LC8 dynein light chain". J. Virol. 74 (21): 10212–10216. doi:10.1128/JVI.74.21.10212-10216.2000. PMC 102061. PMID 11024151.
- ↑ "Rabies". University of Northern British Columbia. Retrieved 2008-10-10.
- ↑ Taylor PJ (December 1993). "A systematic and population genetic approach to the rabies problem in the yellow mongoose (Cynictis penicillata)". Onderstepoort J. Vet. Res. 60 (4): 379–87. PMID 7777324.
- ↑ Benmansour A (1991). "Antigenicity of rabies virus glycoprotein". Journal of Virology. 65 (8): 4198–4203. PMC 248855. PMID 1712859.
- ↑ Marissen, WE.; Kramer, RA.; Rice, A.; Weldon, WC.; Niezgoda, M.; Faber, M.; Slootstra, JW.; Meloen, RH.; et al. (Apr 2005). "Novel rabies virus-neutralizing epitope recognized by human monoclonal antibody: fine mapping and escape mutant analysis". J Virol. 79 (8): 4672–8. doi:10.1128/JVI.79.8.4672-4678.2005. PMC 1069557. PMID 15795253.
- ↑ Wiktor, TJ.; György, E.; Schlumberger, D.; Sokol, F.; Koprowski, H. (Jan 1973). "Antigenic properties of rabies virus components". J Immunol. 110 (1): 269–76. PMID 4568184.
- ↑ Bakker, AB.; Marissen, WE.; Kramer, RA.; Rice, AB.; Weldon, WC.; Niezgoda, M.; Hanlon, CA.; Thijsse, S.; et al. (Jul 2005). "Novel human monoclonal antibody combination effectively neutralizing natural rabies virus variants and individual in vitro escape mutants". J Virol. 79 (14): 9062–8. doi:10.1128/JVI.79.14.9062-9068.2005. PMC 1168753. PMID 15994800.
- ↑ Shannon LM, Poulton JL, Emmons RW, Woodie JD, Fowler ME (April 1988). "Serological survey for rabies antibodies in raptors from California". J. Wildl. Dis. 24 (2): 264–7. doi:10.7589/0090-3558-24.2.264. PMID 3286906.
- ↑ Gough PM, Jorgenson RD (1976). "Rabies antibodies in sera of wild birds". Journal of Wildlife Diseases. 12 (3): 392–5. doi:10.7589/0090-3558-12.3.392. PMID 16498885.
- ↑ Jorgenson RD, Gough PM (July 1976). "Experimental rabies in a great horned owl". J. Wildl. Dis. 12 (3): 444–7. doi:10.7589/0090-3558-12.3.444.
- ↑ Wong, Derek. "Rabies". Wong's Virology. Retrieved 19 Mar 2009.
- ↑ Campbell, James B.; Charlton, K.M. (1988). Developments in Veterinary Virology: Rabies. Springer. p. 48. ISBN 0-89838-390-0.
- ↑ Pawan JL (1959). "The transmission of paralytic rabies in Trinidad by the vampire bat (Desmodus rotundus murinus Wagner". Caribbean Medical Journal. 21: 110–36. PMID 13858519.
- ↑ Pawan JL (1959). "Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection". Caribbean Medical Journal. 21: 137–56. PMID 14431118.
- ↑ Taylor PJ (December 1993). "A systematic and population genetic approach to the rabies problem in the yellow mongoose (Cynictis penicillata)". The Onderstepoort Journal of Veterinary Research. 60 (4): 379–87. PMID 7777324.
- ↑ "Rabies. Other Wild Animals: Terrestrial carnivores: raccoons, skunks and foxes". Centers for Disease Control and Prevention(CDC). Retrieved 2010-12-23.
- ↑ McRuer DL, Jones KD (May 2009). "Behavioral and nutritional aspects of the Virginian opossum (Didelphis virginiana)". The veterinary clinics of North America. Exotic animal practice. 12 (2): 217–36, viii. doi:10.1016/j.cvex.2009.01.007. PMID 19341950.
- ↑ The Merck Manual, 11th Edition (1983), p. 183
- ↑ The Merck manual of Medical Information. Second Home Edition, (2003), p. 484.
- ↑ Turton, Jenny (2000). "Rabies: a killer disease". National Department of Agriculture.
- ↑ Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR (March 2005). "Transmission of rabies virus from an organ donor to four transplant recipients" (PDF). N Engl J Med. 352 (11): 1103–11. doi:10.1056/NEJMoa043018. PMID 15784663.
- ↑ Jackson, Alan C., Wunner, William H. (2002). Rabies. Academic Press. p. 290. ISBN 978-0-12-379077-4.
- ↑ Joanne Lynn, M.D. (October 1997) Transverse Myelitis: Symptoms, Causes and Diagnosis The Transverse Myelitis Association
- ↑ Larry Ernest Davis; Molly K. King; Jessica L. Schultz (15 June 2005). Fundamentals of neurologic disease. Demos Medical Publishing. p. 73. ISBN 978-1-888799-84-2.
- ↑ Nadin-Davis, S. A.; Real, L. A. (2011). "Molecular phylogenetics of the lyssaviruses--insights from a coalescent approach". Adv Virus Res. Advances in Virus Research. 79: 203–238. doi:10.1016/B978-0-12-387040-7.00011-1. ISBN 9780123870407. PMID 21601049.
- ↑ McElhinney, L. M.; Marston, D. A.; Stankov, S; Tu, C.; Black, C.; Johnson, N.; Jiang, Y.; Tordo, N.; Müller, T.; Fooks, A. R. (2008). "Molecular epidemiology of lyssaviruses in Eurasia". Dev Biol (Basel). 131: 125–131. PMID 18634471.
- ↑ Kuzmina, N. A.; Kuzmin, I. V.; Ellison, J. A.; Taylor, S. T.; Bergman, D. L.; Dew, B.; Rupprecht, C. E. (2013). "A reassessment of the evolutionary timescale of bat rabies viruses based upon glycoprotein gene sequences". Virus Genes. Forthcoming (2): 305. doi:10.1007/s11262-013-0952-9.
- ↑ Ginger, M., Haberl M., Conzelmann K.-K., Schwarz M. and Frick A. (2013). Revealing the secrets of neuronal circuits with recombinant rabies virus technology. Front. Neural Circuits. doi:10.3389/fncir.2013.00002