Lurasidone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 49: | Line 49: | ||
|offLabelPedGuideSupport=*safety and effectiveness not established in pediatric patients. | |offLabelPedGuideSupport=*safety and effectiveness not established in pediatric patients. | ||
|offLabelPedNoGuideSupport=*safety and effectiveness not established in pediatric patients. | |offLabelPedNoGuideSupport=*safety and effectiveness not established in pediatric patients. | ||
|contraindications=*Known hypersensitivity to lurasidone HCl or any components in the formulation. [[Angioedema]] has been observed with lurasidone. | |contraindications=*Known hypersensitivity to lurasidone HCl or any components in the formulation. [[Angioedema]] has been observed with [[lurasidone]]. | ||
*Strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.). | *Strong CYP3A4 inhibitors (e.g., [[ketoconazole]], [[clarithromycin]], [[ritonavir]], [[voriconazole]], [[mibefradil]], etc.). | ||
*Strong[[ CYP3A4 inducers]] (e.g., rifampin, avasimibe, St. John's wort, phenytoin, carbamazepine, etc.). | *Strong[[ CYP3A4 inducers]] (e.g., [[rifampin]], [[avasimibe]], [[St. John's wort]], [[phenytoin]], [[carbamazepine]], etc.). | ||
|warnings=[[File:Latudawarning.png|600pnxpx|thumbnail|left]] | |warnings=[[File:Latudawarning.png|600pnxpx|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
| Line 457: | Line 457: | ||
| StdInChIKey=PQXKDMSYBGKCJA-CVTJIBDQSA-N | | StdInChIKey=PQXKDMSYBGKCJA-CVTJIBDQSA-N | ||
}} | }} | ||
|mechAction=*The mechanism of action of LATUDA in the treatment of [[schizophrenia]] and [[bipolar depression]] is unknown. However, its efficacy in schizophrenia and bipolar depression could be mediated through a combination of central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism. | |mechAction=*The mechanism of action of LATUDA in the treatment of [[schizophrenia]] and [[bipolar depression]] is unknown. However, its efficacy in schizophrenia and bipolar depression could be mediated through a combination of central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism. | ||
|structure=*LATUDA is an atypical antipsychotic belonging to the chemical class of benzisothiazol derivatives. | |structure=*LATUDA is an atypical antipsychotic belonging to the chemical class of benzisothiazol derivatives. | ||
Revision as of 15:06, 9 September 2014
{{DrugProjectFormSinglePage |authorTag=Kiran Singh, M.D. [1] |genericName=lurasidone |aOrAn=an |drugClass=atypical antipsychotic |indicationType=treatment |indication=schizophrenia and bipolar depression |hasBlackBoxWarning=Yes |adverseReactions=somnolence, akathisia, extrapyramidal symptoms, and nausea |blackBoxWarningTitle=WARNINGS: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS; AND SUICIDAL THOUGHTS AND BEHAVIORS |blackBoxWarningBody=* Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
- LATUDA is not approved for use in patients with dementia-related psychosis.
- Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older.
- In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber.
|fdaLIADAdult=====Schizophrenia====
- LATUDA is indicated for the treatment of patients with schizophrenia.
- The efficacy of LATUDA in schizophrenia was established in five 6-week controlled studies of adult patients with schizophrenia.
- The effectiveness of LATUDA for longer-term use, that is, for more than 6 weeks, has not been established in controlled studies. Therefore, the physician who elects to use LATUDA for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
Depressive Episodes Associated with Bipolar I Disorder
- Monotherapy: LATUDA is indicated as monotherapy for the treatment of patients with major depressive episodes associated with bipolar I disorder (bipolar depression). The efficacy of LATUDA was established in a 6-week monotherapy study in adult patients with bipolar depression.
- Adjunctive Therapy with Lithium or Valproate: LATUDA is indicated as adjunctive therapy with either lithium or valproate for the treatment of patients with major depressive episodes associated with bipolar I disorder (bipolar depression). The efficacy of LATUDA as adjunctive therapy was established in a 6-week study in adult patients with bipolar depression who were treated with lithium or valproate.
- The effectiveness of LATUDA for longer-term use, that is, for more than 6 weeks, has not been established in controlled studies. Therefore, the physician who elects to use LATUDA for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
- The efficacy of LATUDA in the treatment of mania associated withbipolar disorder has not been established.
Dosing Information
Schizophrenia
- The recommended starting dose of LATUDA is 40 mg once daily. Initial dose titration is not required. LATUDA has been shown to be effective in a dose range of 40 mg per day to 160 mg per day [see Clinical Studies (14.1)]. The maximum recommended dose is 160 mg per day.
Depressive Episodes Associated with Bipolar I Disorder
- The recommended starting dose of LATUDA is 20 mg given once daily as monotherapy or as adjunctive therapy with lithium or valproate. Initial dose titration is not required. LATUDA has been shown to be effective in a dose range of 20 mg per day to 120 mg per day as monotherapy or as adjunctive therapy with lithium or valproate. The maximum recommended dose, as monotherapy or as adjunctive therapy with lithium or valproate, is 120 mg per day. In the monotherapy study, the higher dose range (80 mg to 120 mg per day) did not provide additional efficacy, on average, compared to the lower dose range (20 to 60 mg per day)
Dose Modifications Due to Drug Interactions
- Concomitant Use with CYP3A4 Inhibitors
- LATUDA should not be used concomitantly with a strong CYP3A4 inhibitor (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.)
- If LATUDA is being prescribed and a moderate CYP3A4 inhibitor (e.g. diltiazem, atazanavir, erythromycin, fluconazole, verapamil etc.) is added to the therapy, the LATUDA dose should be reduced to half of the original dose level. Similarly, if a moderate CYP3A4 inhibitor is being prescribed and LATUDA is added to the therapy, the recommended starting dose of LATUDA is 20 mg per day, and the maximum recommended dose of LATUDA is 80 mg per day.
- Grapefruit and grapefruit juice should be avoided in patients taking LATUDA, since these may inhibit CYP3A4 and alter LATUDA concentrations.
- Concomitant Use with CYP3A4 Inducers
- LATUDA should not be used concomitantly with a strong CYP3A4 inducer (e.g., rifampin, avasimibe, St. John's wort, phenytoin, carbamazepine, etc.) . If LATUDA is used concomitantly with a moderate CYP3A4 inducer, it may be necessary to increase the LATUDA dose after chronic treatment (7 days or more) with the CYP3A4 inducer.
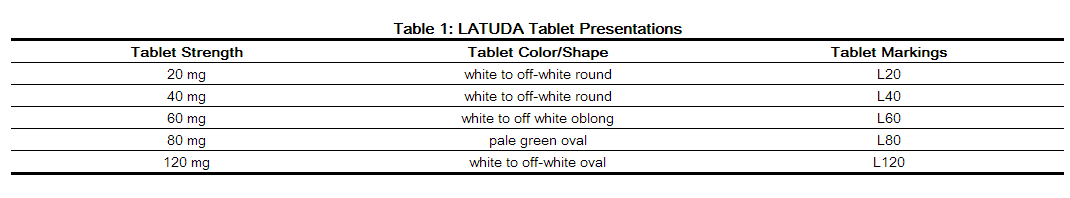
DOSAGE FORMS AND STRENGTHS
- LATUDA tablets are available in the following shape and color (Table 1) with respective one-sided debossing:
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Lurasidone in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Lurasidone in adult patients.
|fdaLIADPed=*Safety and effectiveness in pediatric patients have not been established.
|offLabelPedGuideSupport=*safety and effectiveness not established in pediatric patients.
|offLabelPedNoGuideSupport=*safety and effectiveness not established in pediatric patients.
|contraindications=*Known hypersensitivity to lurasidone HCl or any components in the formulation. Angioedema has been observed with lurasidone.
- Strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.).
- StrongCYP3A4 inducers (e.g., rifampin, avasimibe, St. John's wort, phenytoin, carbamazepine, etc.).
|warnings=
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
- Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6- to 1.7-times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. LATUDA is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
Suicidal Thoughts and Behaviors in Adolescents and Young Adults
- Patients withmajor depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.
- Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
- The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk of differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 2.
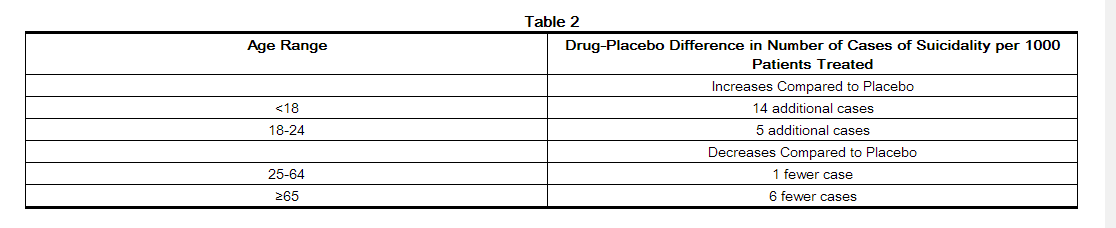
- No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
- It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
- All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
- The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
- Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
- Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidal thoughts and behaviors, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for LATUDA should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
Cerebrovascular Adverse Reactions, Including Stroke in Elderly Patients with Dementia-Related Psychosis
- In placebo-controlled trials with risperidone, aripiprazole, and olanzapine in elderly subjects with dementia, there was a higher incidence of cerebrovascular adverse reactions (cerebrovascular accidents and transient ischemic attacks), including fatalities, compared to placebo-treated subjects. LATUDA is not approved for the treatment of patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome
- A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including LATUDA.
- Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure,tachycardia, diaphoresis, andcardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
- The diagnostic evaluation of patients with this syndrome is complicated. It is important to exclude cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
- The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; 2) intensive symptomatic treatment and medical monitoring; and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
- If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. If reintroduced, the patient should be carefully monitored, since recurrences of NMS have been reported.
Tardive Dyskinesia
- Tardive dyskinesia is a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements that can develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to causetardive dyskinesia is unknown.
- The risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
- There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
- Given these considerations, LATUDA should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that (1) is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
- If signs and symptoms of tardive dyskinesia appear in a patient on LATUDA, drug discontinuation should be considered. However, some patients may require treatment with LATUDA despite the presence of the syndrome.
Metabolic Changes
- Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
- Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of treatment-emergent hyperglycemia-related adverse events in patients treated with the atypical antipsychotics. Because LATUDA was not marketed at the time these studies were performed, it is not known if LATUDA is associated with this increased risk.
- Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria' polyphagia, and weakness. Patients who develop symptoms ofhyperglycemiaduring treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
Schizophrenia
- Pooled data from short-term, placebo-controlled schizophrenia studies are presented in Table 3
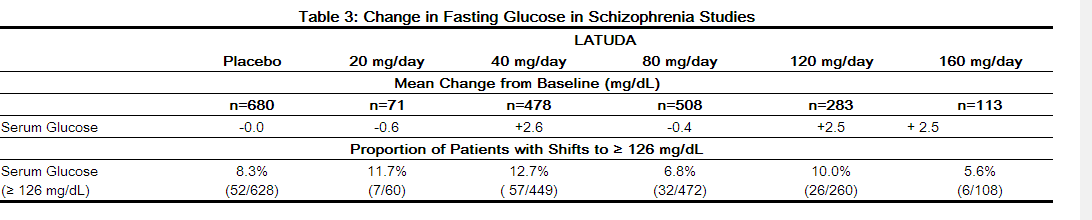
- In the uncontrolled, longer-term schizophrenia studies (primarily open-label extension studies), LATUDA was associated with a mean change in glucose of +1.8 mg/dL at week 24 (n=355), +0.8 mg/dL at week 36 (n=299) and +2.3 mg/dL at week 52 (n=307).
Bipolar Depression
- Monotherapy
- Data from the short-term, flexible-dose, placebo-controlled monotherapy bipolar depression study are presented in Table 4.
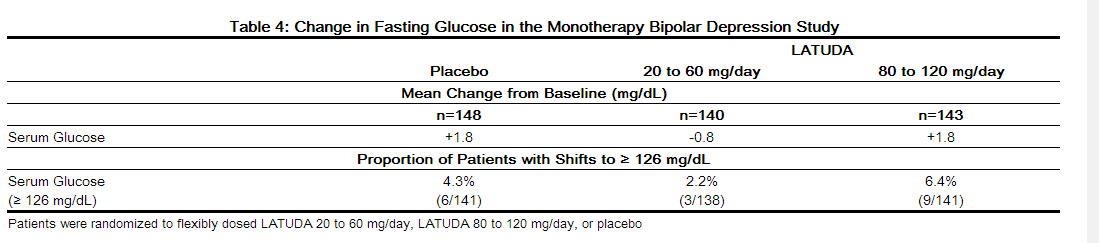
- In the uncontrolled, open-label, longer-term bipolar depression study, patients who received LATUDA as monotherapy in the short-term study and continued in the longer-term study, had a mean change in glucose of +1.2 mg/dL at week 24 (n=129).
- Adjunctive Therapy with Lithium or Valproate
- Data from the short-term, flexible-dosed, placebo-controlled adjunctive therapy bipolar depression studies are presented in Table 5.
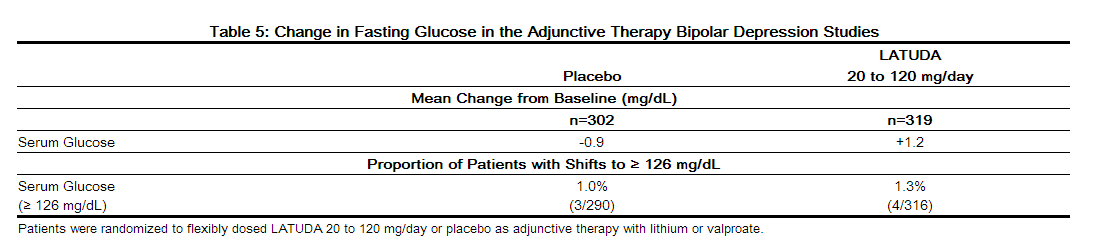
- In the uncontrolled, open-label, longer-term bipolar depression study, patients who received LATUDA as adjunctive therapy with either lithium or valproate in the short-term study and continued in the longer-term study, had a mean change in glucose of +1.7 mg/dL at week 24 (n=88).
Dyslipidemia
- Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
Schizophrenia
- Pooled data from short-term, placebo-controlled schizophrenia studies are presented in Table 6.
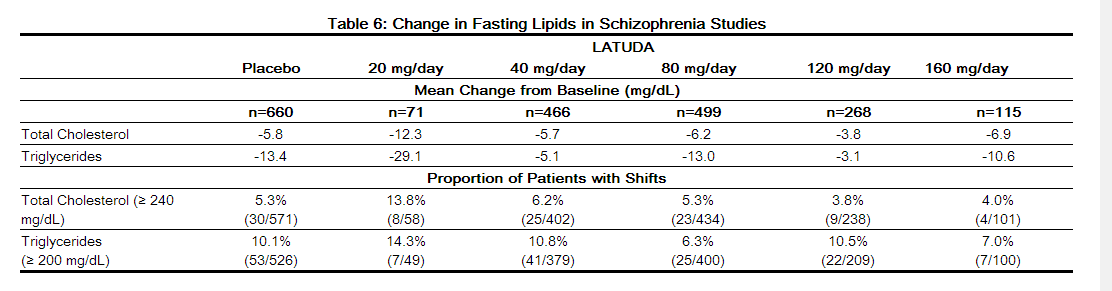
- In the uncontrolled, longer-term schizophrenia studies (primarily open-label extension studies), LATUDA was associated with a mean change in total cholesterol and triglycerides of -3.8 (n=356) and -15.1 (n=357) mg/dL at week 24, -3.1 (n=303) and -4.8 (n=303) mg/dL at week 36 and -2.5 (n=307) and -6.9 (n=307) mg/dL at week 52, respectively.
Bipolar Depression
- Monotherapy
Data from the short-term, flexible-dosed, placebo-controlled, monotherapy bipolar depression study are presented in Table 7
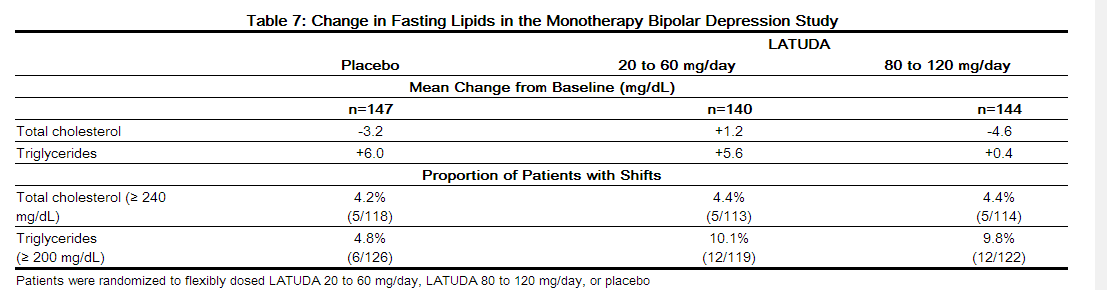
- In the uncontrolled, open-label, longer-term bipolar depression study, patients who received LATUDA as monotherapy in the short-term and continued in the longer-term study had a mean change in total cholesterol and triglycerides of -0.5 (n=130) and -1.0 (n=130) mg/dL at week 24, respectively.
- Adjunctive Therapy with Lithium or Valproate
- Data from the short-term, flexible-dosed, placebo-controlled, adjunctive therapy bipolar depression studies are presented in Table 8.
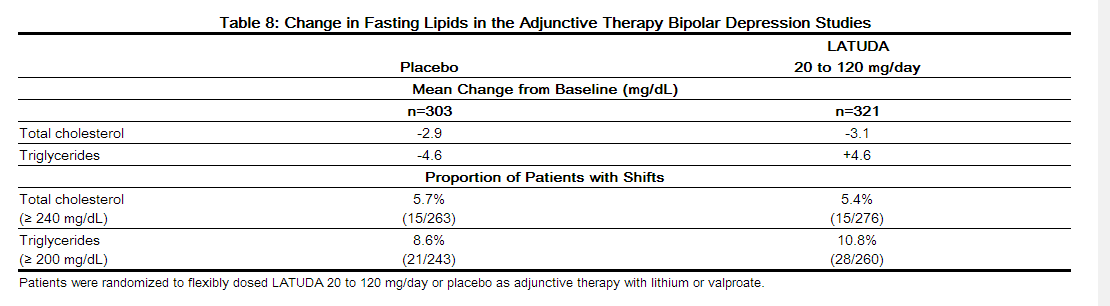
- In the uncontrolled, open-label, longer-term bipolar depression study, patients who received LATUDA, as adjunctive therapy with either lithium or valproate in the short-term study and continued in the longer-term study, had a mean change in total cholesterol and triglycerides of -0.9 (n=88) and +5.3 (n=88) mg/dL at week 24, respectively.
Weight Gain
- Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
Schizophrenia
- Pooled data from short-term, placebo-controlled schizophrenia studies are presented in Table 9. The mean weight gain was +0.43 kg for LATUDA-treated patients compared to -0.02 kg for placebo-treated patients. Change in weight from baseline for olanzapine was +4.15 kg and for quetiapine extended-release was +2.09 kg in Studies 3 and 5 , respectively. The proportion of patients with a ≥ 7% increase in body weight (at Endpoint) was 4.8% for LATUDA-treated patients versus 3.3% for placebo-treated patients.
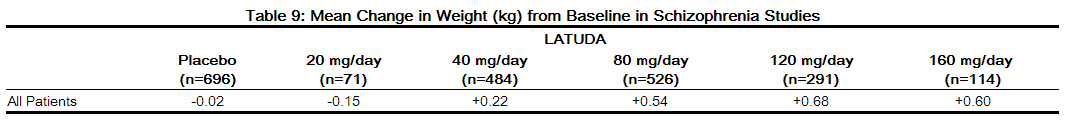
- In the uncontrolled, longer-term schizophrenia studies (primarily open-label extension studies), LATUDA was associated with a mean change in weight of -0.69 kg at week 24 (n=755), -0.59 kg at week 36 (n=443) and -0.73 kg at week 52 (n=377).
Bipolar Depression
- Monotherapy
- Data from the short-term, flexible-dosed, placebo-controlled monotherapy bipolar depression study are presented in Table 10. The mean weight gain was +0.29 kg for LATUDA-treated patients compared to -0.04 kg for placebo-treated patients. The proportion of patients with a ≥ 7% increase in body weight (at Endpoint) was 2.4% for LATUDA-treated patients versus 0.7% for placebo-treated patients.

- In the uncontrolled, open-label, longer-term bipolar depression study, patients who received LATUDA as monotherapy in the short-term and continued in the longer-term study had a mean change in weight of -0.02 kg at week 24 (n=130).
Adjunctive Therapy with Lithium or Valproate
- Data from the short-term, flexible-dosed, placebo-controlled adjunctive therapy bipolar depression studies are presented in Table 11. The mean weight gain was +0.11 kg for LATUDA-treated patients compared to +0.16 kg for placebo-treated patients. The proportion of patients with a ≥ 7% increase in body weight (at Endpoint) was 3.1% for LATUDA-treated patients versus 0.3% for placebo-treated patients.

- In the uncontrolled, open-label, longer-term bipolar depression study, patients who were treated with LATUDA, as adjunctive therapy with either lithium or valproate in the short-term and continued in the longer-term study, had a mean change in weight of +1.28 kg at week 24 (n=86).
Hyperprolactinemia
- As with other drugs that antagonize dopamine D2 receptors, LATUDA elevates prolactin levels.
- Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotrophin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea,amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating compounds. Long-standing hyperprolactinemia, when associated with hypogonadism, may lead to decreased bone density in both female and male patients.
- Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of these drugs is considered in a patient with previously detected breast cancer. As is common with compounds which increase prolactin release, an increase in mammary gland neoplasia was observed in a LATUDA carcinogenicity study conducted in rats and mice . Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive.
Schizophrenia
- In short-term, placebo-controlled schizophrenia studies, the median change from baseline to endpoint in prolactin levels for LATUDA-treated patients was +0.4 ng/mL and was -1.9 ng/mL in the placebo-treated patients. The median change from baseline to endpoint for males was +0.5 ng/mL and for females was -0.2 ng/mL. Median changes for prolactin by dose are shown in Table 12.
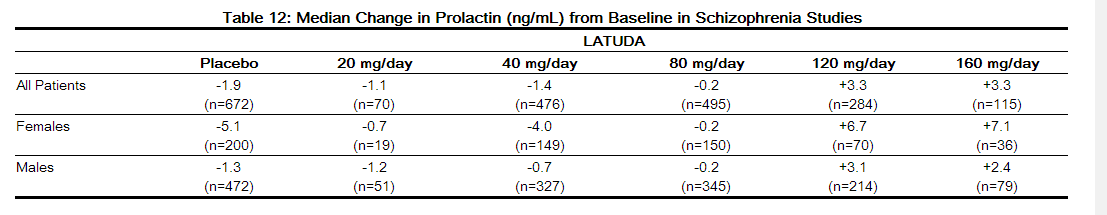
- The proportion of patients with prolactin elevations ≥ 5× upper limit of normal (ULN) was 2.8% for LATUDA-treated patients versus 1.0% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥ 5x ULN was 5.7% for LATUDA-treated patients versus 2.0% for placebo-treated female patients. The proportion of male patients with prolactin elevations ≥ 5x ULN was 1.6% versus 0.6% for placebo-treated male patients.
- In the uncontrolled longer-term schizophrenia studies (primarily open-label extension studies), LATUDA was associated with a median change in prolactin of -0.9 ng/mL at week 24 (n=357), -5.3ng/mL at week 36 (n=190) and -2.2 ng/mL at week 52 (n=307).
Bipolar Depression
- Monotherapy
- The median change from baseline to endpoint in prolactin levels, in the short-term, flexible-dosed, placebo-controlled monotherapy bipolar depression study, was +1.7 ng/mL and +3.5 ng/mL with LATUDA 20 to 60 mg/day and 80 to 120 mg/day, respectively compared to +0.3 ng/mL with placebo-treated patients. The median change from baseline to endpoint for males was +1.5 ng/mL and for females was +3.1 ng/mL. Median changes for prolactin by dose range are shown in Table 13.
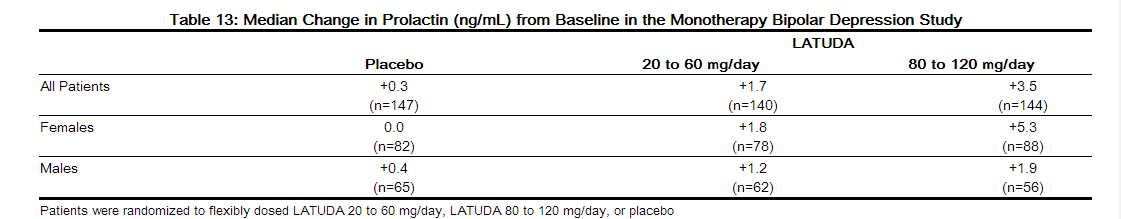
- The proportion of patients with prolactin elevations ≥ 5x upper limit of normal (ULN) was 0.4% for LATUDA-treated patients versus 0.0% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥ 5x ULN was 0.6% for LATUDA-treated patients versus 0% for placebo-treated female patients. The proportion of male patients with prolactin elevations ≥ 5x ULN was 0% versus 0% for placebo-treated male patients.
- In the uncontrolled, open-label, longer-term bipolar depression study, patients who were treated with LATUDA as monotherapy in the short-term and continued in the longer-term study, had a median change in prolactin of -1.15 ng/mL at week 24 (n=130).
- Adjunctive Therapy with Lithium or Valproate
- The median change from baseline to endpoint in prolactin levels, in the short-term, flexible-dosed, placebo-controlled adjunctive therapy bipolar depression studies was +2.8 ng/mL with LATUDA 20 to 120 mg/day compared to 0.0 ng/mL with placebo-treated patients. The median change from baseline to endpoint for males was +2.4 ng/mL and for females was +3.2 ng/mL. Median changes for prolactin across the dose range are shown in Table 14
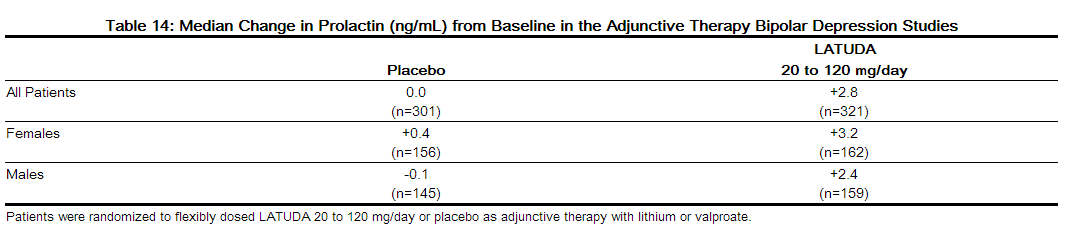
- The proportion of patients with prolactin elevations ≥ 5x upper limit of normal (ULN) was 0.0% for LATUDA-treated patients versus 0.0% for placebo-treated patients. The proportion of female patients with prolactin elevations ≥ 5x ULN was 0% for LATUDA-treated patients versus 0% for placebo-treated female patients. The proportion of male patients with prolactin elevations ≥ 5x ULN was 0% versus 0% for placebo-treated male patients.
- In the uncontrolled, open-label, longer-term bipolar depression study, patients who were treated with LATUDA, as adjunctive therapy with either lithium or valproate, in the short-term and continued in the longer-term study, had a median change in prolactin of -2.9 ng/mL at week 24 (n=88).
Leukopenia, Neutropenia and Agranulocytosis
- Leukopenia/neutropenia has been reported during treatment with antipsychotic agents. Agranulocytosis (including fatal cases) has been reported with other agents in the class.
- Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and history of drug-induced leukopenia/neutropenia. Patients with a pre-existing low WBC or a history of drug-induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and LATUDA should be discontinued at the first sign of decline in WBC, in the absence of other causative factors.
- Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count < 1000/mm3) should discontinue LATUDA and have their WBC followed until recovery.
Orthostatic Hypotension and Syncope
- LATUDA may cause orthostatic hypotension and syncope, perhaps due to its α1-adrenergic receptor antagonism. Associated adverse reactions can include dizziness, lightheadedness, tachycardia, and bradycardia. Generally, these risks are greatest at the beginning of treatment and during dose escalation. Patients at increased risk of these adverse reactions or at increased risk of developing complications from hypotension include those with dehydration, hypovolemia, treatment with antihypertensive medication, history of cardiovascular disease (e.g., heart failure, myocardial infarction, ischemia, or conduction abnormalities), history of cerebrovascular disease, as well as patients who are antipsychotic-naïve. In such patients, consider using a lower starting dose and slower titration, and monitor orthostatic vital signs.
- Orthostatic hypotension, as assessed by vital sign measurement, was defined by the following vital sign changes: ≥ 20 mm Hg decrease in systolic blood pressure and ≥ 10 bpm increase in pulse from sitting to standing or supine to standing position.
Schizophrenia
- The incidence of orthostatic hypotension and syncope reported as adverse events from short-term, placebo-controlled schizophrenia studies was (LATUDA incidence, placebo incidence): orthostatic hypotension [0.3% (5/1508), 0.1% (1/708)] and syncope [0.1% (2/1508), 0% (0/708)].
- In short-term schizophrenia clinical studies, orthostatic hypotension, as assessed by vital signs, occurred with a frequency of 0.8% with LATUDA 40 mg, 2.1% with LATUDA 80 mg, 1.7% with LATUDA 120 mg and 0.8% with LATUDA 160 mg compared to 0.7% with placebo.
Bipolar Depression
- Monotherapy
- In the short-term, flexible-dose, placebo-controlled monotherapy bipolar depression study, there were no reported adverse events of orthostatic hypotension and syncope.
- Orthostatic hypotension, as assessed by vital signs, occurred with a frequency of 0.6% with LATUDA 20 to 60 mg and 0.6% with LATUDA 80 to 120 mg compared to 0% with placebo.
- Adjunctive Therapy with Lithium or Valproate
- In the short-term, flexible-dose, placebo-controlled adjunctive therapy bipolar depression therapy studies, there were no reported adverse events of orthostatic hypotension and syncope. Orthostatic hypotension, as assessed by vital signs, occurred with a frequency of 1.1% with LATUDA 20 to 120 mg compared to 0.9% with placebo
Seizures
- As with other antipsychotic drugs, LATUDA should be used cautiously in patients with a history of seizures or with conditions that lower the seizure threshold, e.g., Alzheimer's dementia. Conditions that lower the seizure threshold may be more prevalent in patients 65 years or older.
Schizophrenia
- In short-term, placebo-controlled schizophrenia studies, seizures/convulsions occurred in 0.1% (2/1508) of patients treated with LATUDA compared to 0.1% (1/708) placebo-treated patients.
Bipolar Depression
- Monotherapy
- In the short-term, flexible-dose, placebo-controlled monotherapy bipolar depression study, no patient experienced seizures/convulsions.
- Adjunctive Therapy with Lithium or Valproate
- In the short-term, flexible-dose, placebo-controlled adjunctive therapy bipolar depression studies, no patient experienced seizures/convulsions.
Potential for Cognitive and Motor Impairment
- LATUDA, like other antipsychotics, has the potential to impair judgment, thinking or motor skills. Caution patients about operating hazardous machinery, including motor vehicles, until they are reasonably certain that therapy with LATUDA does not affect them adversely.
- In clinical studies with LATUDA, somnolence included: hypersomnia, hypersomnolence, sedation and somnolence.
Schizophrenia
- In short-term, placebo-controlled schizophrenia studies, somnolence was reported by 17.0% (256/1508) of patients treated with LATUDA (15.5% LATUDA 20 mg, 15.6% LATUDA 40 mg, 15.2% LATUDA 80 mg, 26.5% LATUDA 120 mg and 8.3% LATUDA 160 mg/day) compared to 7.1% (50/708) of placebo patients.
=Bipolar Depression
- Monotherapy
- In the short-term, flexible-dosed, placebo-controlled monotherapy bipolar depression study, somnolence was reported by 7.3% (12/164) and 13.8% (23/167) with LATUDA 20 to 60 mg and 80 to120 mg, respectively compared to 6.5% (11/168) of placebo patients.
- Adjunctive Therapy with Lithium or Valproate
- In the short-term, flexible-dosed, placebo-controlled adjunctive therapy bipolar depression studies, somnolence was reported by 11.4% (41/360) of patients treated with LATUDA 20-120 mg compared to 5.1% (17/334) of placebo patients.
Body Temperature Dysregulation
- Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing LATUDA for patients who will be experiencing conditions that may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
Suicide
- The possibility of a suicide attempt is inherent in psychotic illness and close supervision of high-risk patients should accompany drug therapy. Prescriptions for LATUDA should be written for the smallest quantity of tablets consistent with good patient management in order to reduce the risk of overdose.
Schizophrenia
- In short-term, placebo-controlled schizophrenia studies, the incidence of treatment-emergent suicidal ideation was 0.4% (6/1508) for LATUDA-treated patients compared to 0.8% (6/708) on placebo. No suicide attempts or completed suicides were reported in these studies.
Bipolar Depression
- Monotherapy
- In the short-term, flexible-dose, placebo-controlled monotherapy bipolar depression study, the incidence of treatment-emergent suicidal ideation was 0.0% (0/331) with LATUDA-treated patients compared to 0.0% (0/168) with placebo-treated patients. No suicide attempts or completed suicides were reported in this study.
- Adjunctive Therapy with Lithium or Valproate
- In the short-term, flexible-dose, placebo-controlled adjunctive therapy bipolar depression studies, the incidence of treatment-emergent suicidal ideation was 1.1% (4/360) for LATUDA-treated patients compared to 0.3% (1/334) on placebo. No suicide attempts or completed suicides were reported in these studies.
Activation of Mania/Hypomania
- Antidepressant treatment can increase the risk of developing a manic or hypomanic episode, particularly in patients with bipolar disorder. Monitor patients for the emergence of such episodes.
- In the bipolar depression monotherapy and adjunctive therapy (with lithium or valproate) studies, less than 1% of subjects in the LATUDA and placebo groups developed manic or hypomanic episodes.
Dysphagia
- Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients, in particular those with advanced Alzheimer's dementia. LATUDA and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia.
Neurological Adverse Reactions in Patients with Parkinson's Disease or Dementia with Lewy Bodies
- Patients with Parkinson's Disease or Dementia with Lewy Bodies are reported to have an increased sensitivity to antipsychotic medication. Manifestations of this increased sensitivity include confusion, obtundation, postural instability with frequent falls, extrapyramidal symptoms, and clinical features consistent with the neuroleptic malignant syndrome.
|clinicalTrials=*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The information below is derived from an integrated clinical study database for LATUDA consisting of 3799 patients exposed to one or more doses of LATUDA for the treatment of schizophrenia and bipolar depression in placebo-controlled studies. This experience corresponds with a total experience of 1250.9 patient-years. A total of 1106 LATUDA-treated patients had at least 24 weeks and 371 LATUDA-treated patients had at least 52 weeks of exposure.
- Adverse events during exposure to study treatment were obtained by general inquiry and voluntarily reported adverse experiences, as well as results from physical examinations, vital signs, ECGs, weights and laboratory investigations. Adverse experiences were recorded by clinical investigators using their own terminology. In order to provide a meaningful estimate of the proportion of individuals experiencing adverse events, events were grouped in standardized categories using MedDRA terminology.
Schizophrenia
- The following findings are based on the short-term, placebo-controlled premarketing studies for schizophrenia in which LATUDA was administered at daily doses ranging from 20 to 160 mg (n=1508).
- Commonly Observed Adverse Reactions:
- The most common adverse reactions (incidence ≥ 5% and at least twice the rate of placebo) in patients treated with LATUDA were somnolence, akathisia, extrapyramidal symptoms, and nausea.
- Adverse Reactions Associated with Discontinuation of Treatment:
- A total of 9.5% (143/1508) LATUDA-treated patients and 9.3% (66/708) of placebo-treated patients discontinued due to adverse reactions. There were no adverse reactions associated with discontinuation in subjects treated with LATUDA that were at least 2% and at least twice the placebo rate.
- Adverse Reactions Occurring at an Incidence of 2% or More in LATUDA-Treated Patients: :*Adverse reactions associated with the use of LATUDA (incidence of 2% or greater, rounded to the nearest percent and LATUDA incidence greater than placebo) that occurred during acute therapy (up to 6 weeks in patients with schizophrenia) are shown in Table 15.
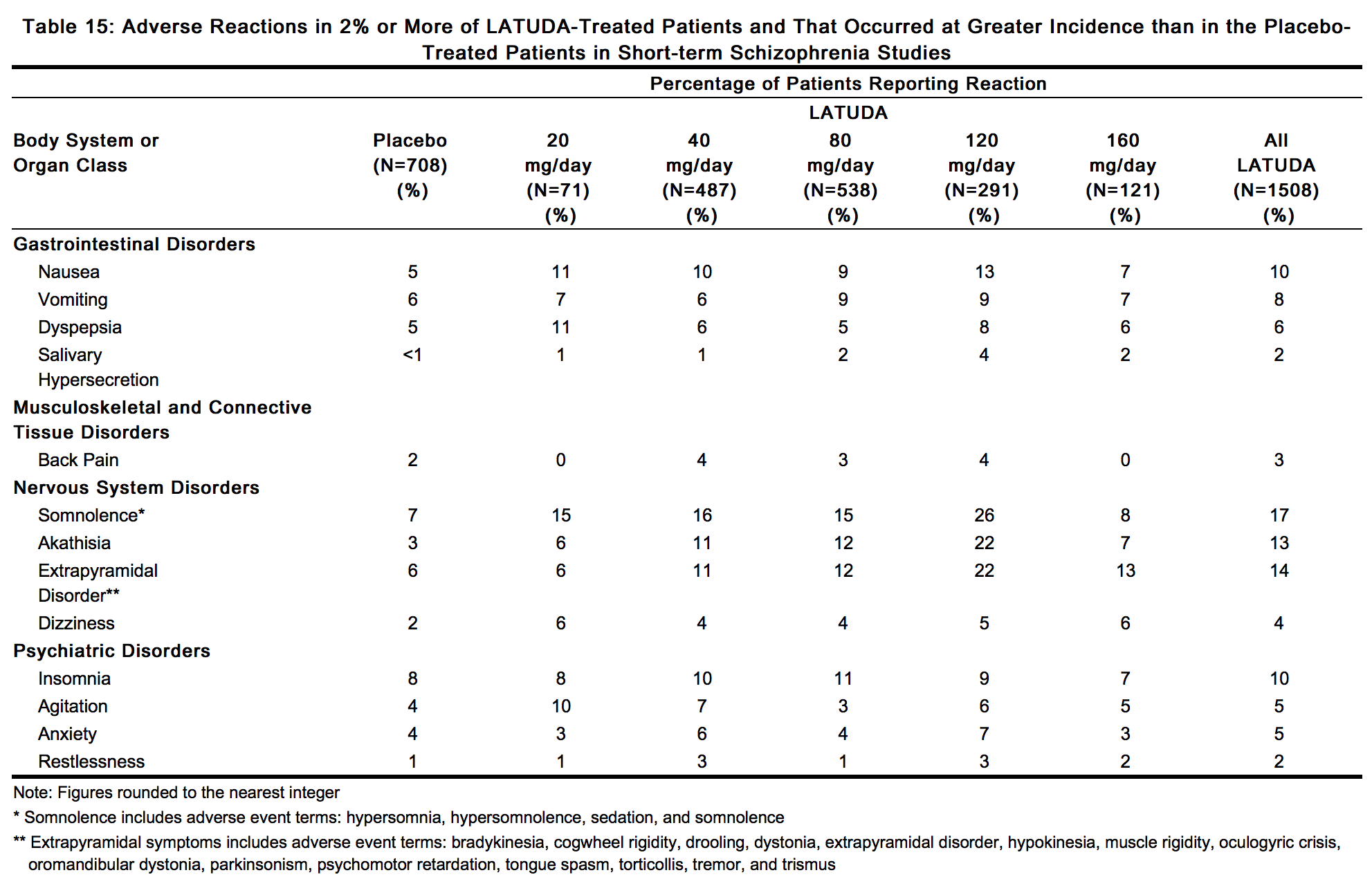
- Dose-Related Adverse Reactions in the Schizophrenia Studies
- Akathisia and extrapyramidal symptoms were dose-related. The frequency of akathisia increased with dose up to 120 mg/day (5.6% for LATUDA 20 mg, 10.7% for LATUDA 40 mg, 12.3% for LATUDA 80 mg, and 22.0% for LATUDA 120 mg). Akathisia was reported by 7.4% (9/121) of patients receiving 160 mg/day. Akathisia occurred in 3.0% of subjects receiving placebo. The frequency of extrapyramidal symptoms increased with dose up to 120 mg/day (5.6% for LATUDA 20 mg, 11.5% for LATUDA 40 mg, 11.9% for LATUDA 80 mg, and 22.0% for LATUDA 120 mg).
Bipolar Depression (Monotherapy)
- The following findings are based on the short-term, placebo-controlled premarketing study for bipolar depression in which LATUDA was administered at daily doses ranging from 20 to 120 mg (n=331).
- Commonly Observed Adverse Reactions:
- The most common adverse reactions (incidence ≥ 5%, in either dose group, and at least twice the rate of placebo) in patients treated with LATUDA were akathisia, extrapyramidal symptoms, somnolence, nausea, vomiting, diarrhea, and anxiety.
- Adverse Reactions Associated with Discontinuation of Treatment:
- A total of 6.0% (20/331) LATUDA-treated patients and 5.4% (9/168) of placebo-treated patients discontinued due to adverse reactions. There were no adverse reactions associated with discontinuation in subjects treated with LATUDA that were at least 2% and at least twice the placebo rate.
- Adverse Reactions Occurring at an Incidence of 2% or More in LATUDA-Treated Patients: :*Adverse reactions associated with the use of LATUDA (incidence of 2% or greater, rounded to the nearest percent and LATUDA incidence greater than placebo) that occurred during acute therapy (up to 6 weeks in patients with bipolar depression) are shown in Table 16.
Table 16: Adverse Reactions in 2% or More of LATU
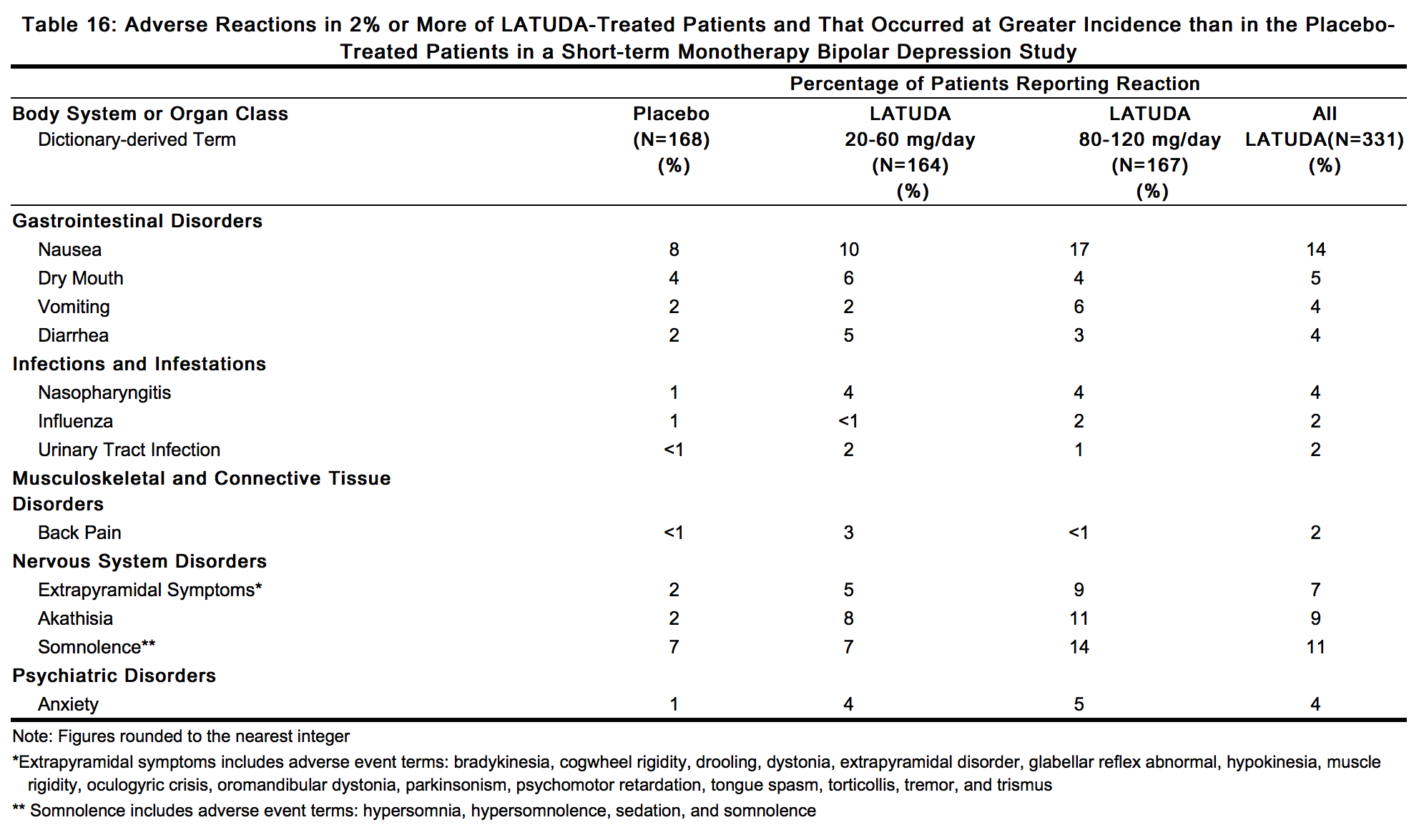
- Dose-Related Adverse Reactions in the Monotherapy Study:
- In the short-term, placebo-controlled study (involving lower and higher LATUDA dose ranges) the adverse reactions that occurred with a greater than 5% incidence in the patients treated with LATUDA in any dose group and greater than placebo in both groups were nausea (10.4%, 17.4%), somnolence (7.3%, 13.8%), akathisia (7.9%, 10.8%), and extrapyramidal symptoms (4.9%, 9.0%) for LATUDA 20 to 60 mg/day and LATUDA 80 to 120 mg/day, respectively.
Bipolar Depression
Adjunctive Therapy with Lithium or Valproate
- The following findings are based on two short-term, placebo-controlled premarketing studies for bipolar depression in which LATUDA was administered at daily doses ranging from 20 to 120 mg as adjunctive therapy with lithium or valproate (n=360).
- Commonly Observed Adverse Reactions:
- The most common adverse reactions (incidence ≥ 5% and at least twice the rate of placebo) in subjects treated with LATUDA were akathisia and somnolence.
- Adverse Reactions Associated with Discontinuation of Treatment:
- A total of 5.8% (21/360) LATUDA-treated patients and 4.8% (16/334) of placebo-treated patients discontinued due to adverse reactions. There were no adverse reactions associated with discontinuation in subjects treated with LATUDA that were at least 2% and at least twice the placebo rate.
- Adverse Reactions Occurring at an Incidence of 2% or More in LATUDA-Treated Patients: :*Adverse reactions associated with the use of LATUDA (incidence of 2% or greater, rounded to the nearest percent and LATUDA incidence greater than placebo) that occurred during acute therapy (up to 6 weeks in patients with bipolar depression) are shown in Table 17.

- Extrapyramidal Symptoms
- Schizophrenia
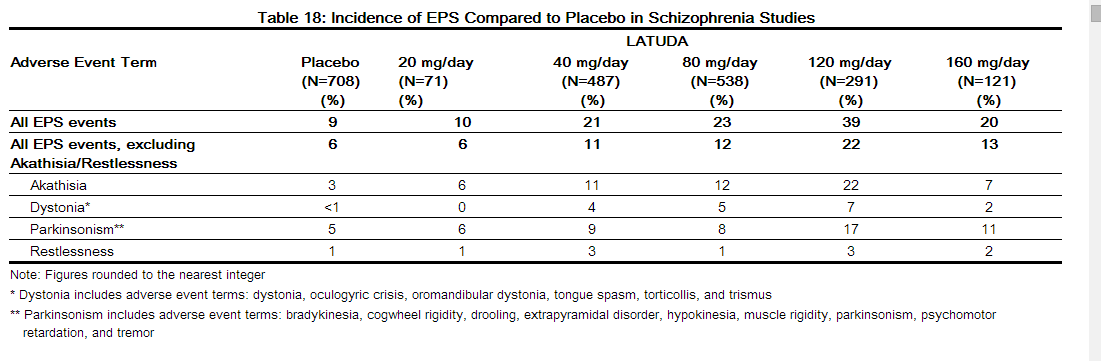
In the short-term, placebo-controlled schizophrenia studies, for LATUDA-treated patients, the incidence of reported events related to extrapyramidal symptoms (EPS), excluding akathisia and restlessness, was 13.5% versus 5.8% for placebo-treated patients. The incidence of akathisia for LATUDA-treated patients was 12.9% versus 3.0% for placebo-treated patients. Incidence of EPS by dose is provided in Table 18.
- Bipolar Depression
- Monotherapy
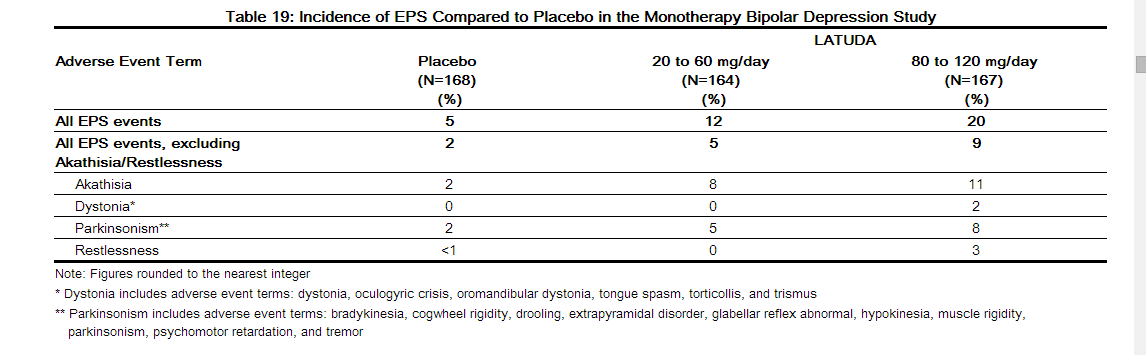
In the short-term, placebo-controlled monotherapy bipolar depression study, for LATUDA-treated patients, the incidence of reported events related to EPS, excluding akathisia and restlessness was 6.9% versus 2.4% for placebo-treated patients. The incidence of akathisia for LATUDA-treated patients was 9.4% versus 2.4% for placebo-treated patients. Incidence of EPS by dose groups is provided in Table 19
- Adjunctive Therapy with Lithium or Valproate
In the short-term, placebo-controlled adjunctive therapy bipolar depression studies, for LATUDA-treated patients, the incidence of EPS, excluding akathisia and restlessness, was 13.9% versus 8.7% for placebo. The incidence of akathisia for LATUDA-treated patients was 10.8% versus 4.8% for placebo-treated patients. Incidence of EPS is provided in Table 20.
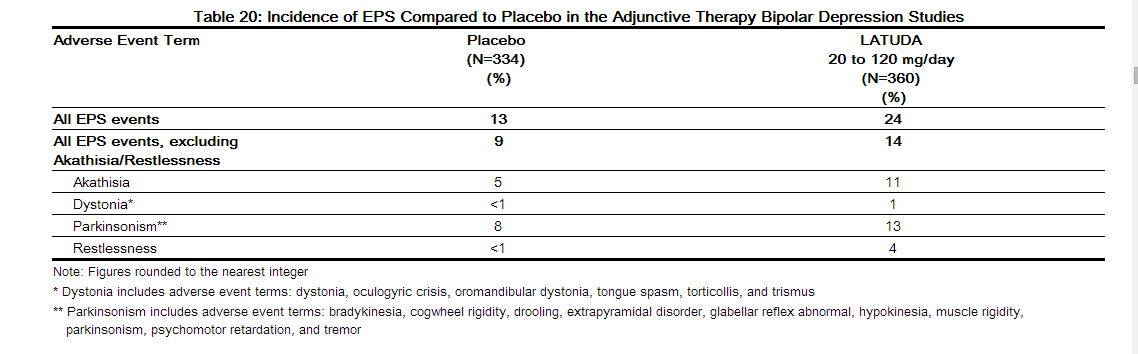
In the short-term, placebo-controlled schizophrenia and bipolar depression studies, data was objectively collected on the Simpson Angus Rating Scale (SAS) for extrapyramidal symptoms (EPS), the Barnes Akathisia Scale (BAS) for akathisia and the Abnormal Involuntary Movement Scale (AIMS) for dyskinesias.
- Schizophrenia
The mean change from baseline for LATUDA-treated patients for the SAS, BAS and AIMS was comparable to placebo-treated patients, with the exception of the Barnes Akathisia Scale global score (LATUDA, 0.1; placebo, 0.0). The percentage of patients who shifted from normal to abnormal was greater in LATUDA-treated patients versus placebo for the BAS (LATUDA, 14.4%; placebo, 7.1%), the SAS (LATUDA, 5.0%; placebo, 2.3%) and the AIMS (LATUDA, 7.4%; placebo, 5.8%).
- Bipolar Depression
- Monotherapy
The mean change from baseline for LATUDA-treated patients for the SAS, BAS and AIMS was comparable to placebo-treated patients. The percentage of patients who shifted from normal to abnormal was greater in LATUDA-treated patients versus placebo for the BAS (LATUDA, 8.4%; placebo, 5.6%), the SAS (LATUDA, 3.7%; placebo, 1.9%) and the AIMS (LATUDA, 3.4%; placebo, 1.2%).
- Adjunctive Therapy with Lithium or Valproate
The mean change from baseline for LATUDA-treated patients for the SAS, BAS and AIMS was comparable to placebo-treated patients. The percentage of patients who shifted from normal to abnormal was greater in LATUDA-treated patients versus placebo for the BAS (LATUDA, 8.7%; placebo, 2.1%), the SAS (LATUDA, 2.8%; placebo, 2.1%) and the AIMS (LATUDA, 2.8%; placebo, 0.6%).
- Dystonia
Class Effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first-generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
- Schizophrenia
In the short-term, placebo-controlled schizophrenia clinical studies, dystonia occurred in 4.2% of LATUDA-treated subjects (0.0% LATUDA 20 mg, 3.5% LATUDA 40 mg, 4.5% LATUDA 80 mg, 6.5% LATUDA 120 mg and 2.5% LATUDA 160 mg) compared to 0.8% of subjects receiving placebo. Seven subjects (0.5%, 7/1508) discontinued clinical trials due to dystonic events – four were receiving LATUDA 80 mg/day and three were receiving LATUDA 120 mg/day.
- Bipolar Depression
Monotherapy In the short-term, flexible-dose, placebo-controlled monotherapy bipolar depression study, dystonia occurred in 0.9% of LATUDA-treated subjects (0.0% and 1.8% for LATUDA 20 to 60 mg/day and LATUDA 80 to 120 mg/day, respectively) compared to 0.0% of subjects receiving placebo. No subject discontinued the clinical study due to dystonic events.
- Adjunctive Therapy with Lithium or Valproate
In the short-term, flexible-dose, placebo-controlled adjunctive therapy bipolar depression studies, dystonia occurred in 1.1% of LATUDA-treated subjects (20 to 120 mg) compared to 0.6% of subjects receiving placebo. No subject discontinued the clinical study due to dystonic events. |postmarketing=*Following is a list of adverse reactions reported by patients treated with LATUDA at multiple doses of ≥ 20 mg once daily within the premarketing database of 2905 patients with schizophrenia. The reactions listed are those that could be of clinical importance, as well as reactions that are plausibly drug-related on pharmacologic or other grounds. Reactions listed in Table 15 or those that appear elsewhere in the LATUDA label are not included. Although the reactions reported occurred during treatment with LATUDA, they were not necessarily caused by it.
- Reactions are further categorized by organ class and listed in order of decreasing frequency according to the following definitions: those occurring in at least 1/100 patients (frequent) (only those not already listed in the tabulated results from placebo-controlled studies appear in this listing); those occurring in 1/100 to 1/1000 patients (infrequent); and those occurring in fewer than 1/1000 patients (rare).
Cardiovascular
- Frequent: tachycardia;
- Infrequent: AV block 1st degree, angina pectoris, bradycardia.
Digestive
- Frequent: abdominal pain, diarrhea;
- Infrequent: gastritis.
Hematologic and Lymphatic
- Infrequent: anemia
Metabolic and Nutritional
- Frequent: decreased appetite
Musculoskeletal
- Rare: rhabdomyolysis
Neurologic
- Infrequent: cerebrovascular accident, dysarthria
Psychiatric Disorders
- Infrequent: abnormal dreams, panic attack, sleep disorder
Skin and Hypersensitivy Reactions
- Frequent: rash, pruritus;
- Rare: angioedema
Ear and Labyrinth Disorders
- Infrequent: vertigo
Eye Disorders
- Frequent: blurred vision
Renal and Urinary Disorders
- Infrequent: dysuria;
- Rare: renal failure
Reproductive System and Breast Disorders
- Infrequent: amenorrhea, dysmenorrhea;
- Rare: breast enlargement, breast pain, galactorrhea, erectile dysfunction
Vascular Disorders
- Frequent: hypertension
Clinical Laboratory Changes
Schizophrenia
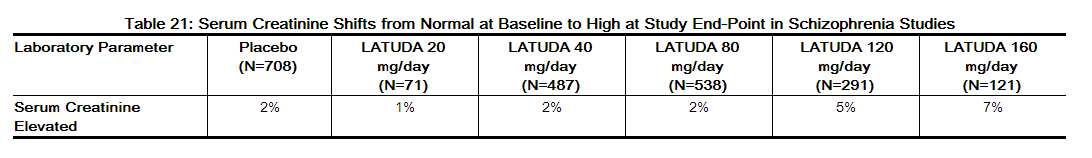
- Serum Creatinine: In short-term, placebo-controlled trials, the mean change from Baseline in serum creatinine was +0.05 mg/dL for LATUDA-treated patients compared to +0.02 mg/dL for placebo-treated patients. A creatinine shift from normal to high occurred in 3.0% (43/1453) of LATUDA-treated patients and 1.6% (11/681) on placebo. The threshold for high creatinine value varied from > 0.79 to > 1.3 mg/dL based on the centralized laboratory definition for each study (Table 21).
Bipolar Depression
- Monotherapy
- Serum Creatinine: In the short-term, flexible-dose, placebo-controlled monotherapy bipolar depression study, the mean change from Baseline in serum creatinine was +0.01 mg/dL for LATUDA-treated patients compared to -0.02 mg/dL for placebo-treated patients. A creatinine shift from normal to high occurred in 2.8% (9/322) of LATUDA-treated patients and 0.6% (1/162) on placebo (Table 22)

- Adjunctive Therapy with Lithium or Valproate
- Serum Creatinine: In short-term, placebo-controlled premarketing adjunctive studies for bipolar depression, the mean change from Baseline in serum creatinine was +0.04 mg/dL for LATUDA-treated patients compared to -0.01 mg/dL for placebo-treated patients. A creatinine shift from normal to high occurred in 4.3% (15/360) of LATUDA-treated patients and 1.6% (5/334) on placebo (Table 23).

|drugInteractions=====Potential for Other Drugs to Affect LATUDA====
- LATUDA is predominantly metabolized by CYP3A4. LATUDA should not be used concomitantly with strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin, ritonavir, voriconazole, mibefradil, etc.) or strong CYP3A4 inducers (e.g., rifampin, avasimibe, St. John's wort, phenytoin, carbamazepine, etc.) [see Contraindications (4)]. The LATUDA dose should be reduced to half of the original level when used concomitantly with moderate inhibitors of CYP3A4 (e.g., diltiazem, atazanavir, erythromycin, fluconazole, verapamil, etc.). If LATUDA is used concomitantly with a moderate CYP3A4 inducer, it may be necessary to increase the LATUDA dose.
Lithium
- It is not necessary to adjust the LATUDA dose when used concomitantly with lithium.
Valproate
- It is not necessary to adjust the LATUDA dose when used concomitantly with valproate. A dedicated drug-drug interaction study has not been conducted with valproate and LATUDA. Based on pharmacokinetic data from the bipolar depression studies valproate levels were not affected by lurasidone, and lurasidone concentrations were not affected by valproate.
Grapefruit
- Grapefruit and grapefruit juice should be avoided in patients taking LATUDA, since these may inhibit CYP3A4 and alter LATUDA concentrations.
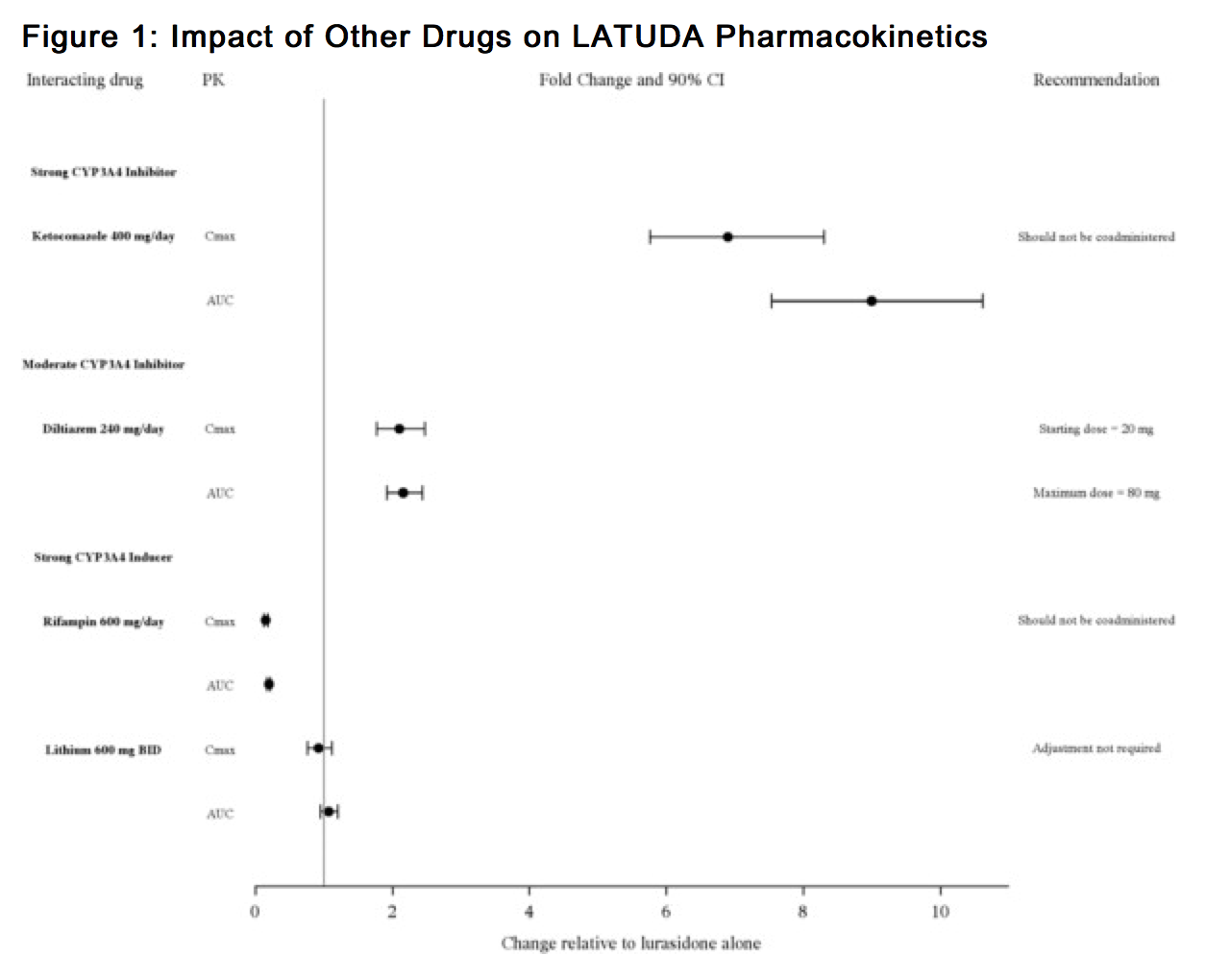
Potential for LATUDA to Affect Other Drugs
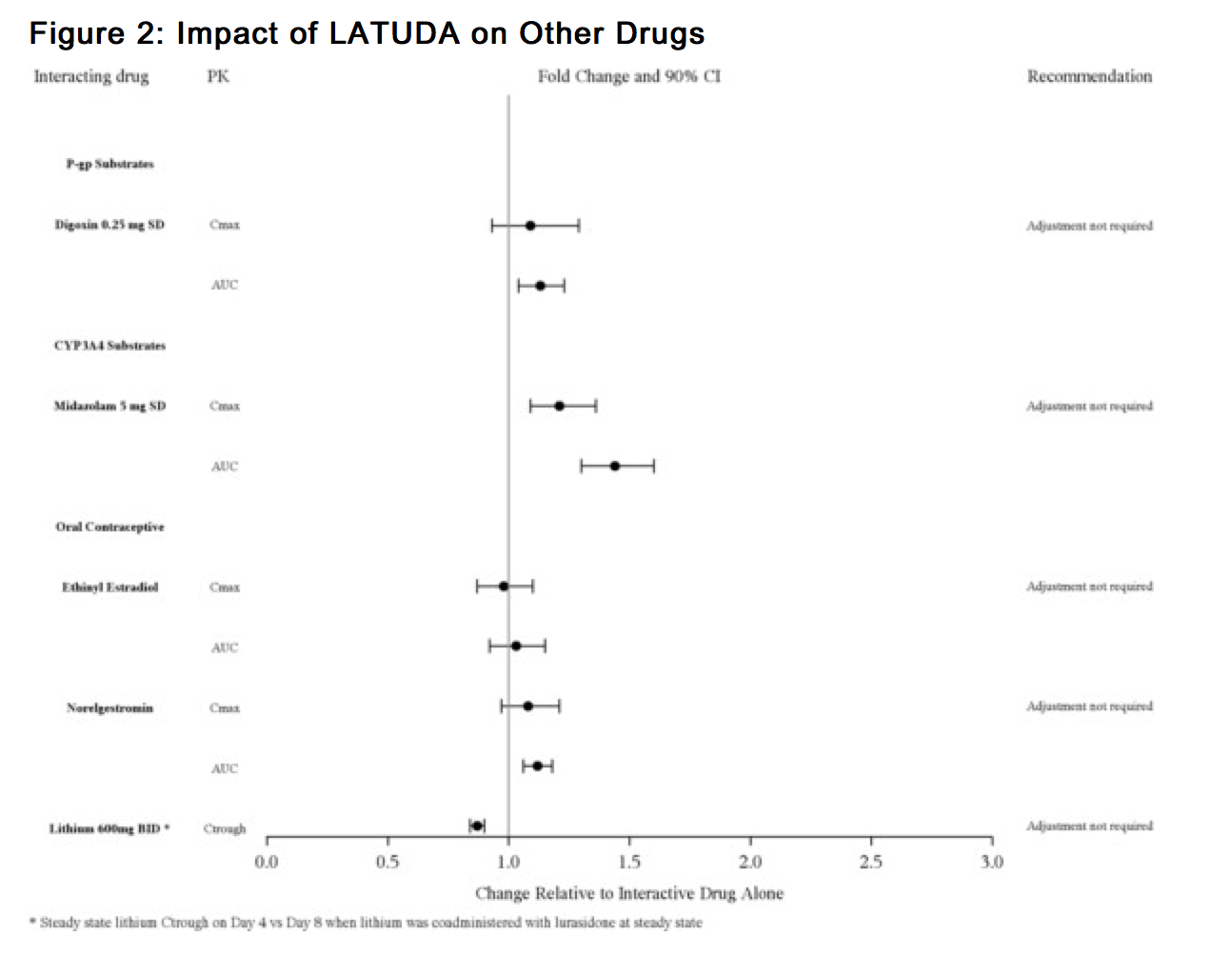
- No dose adjustment is needed for lithium, substrates of P-gp, CYP3A4 (Figure 2) or valproate when coadministered with LATUDA. ).
|useInPregnancyFDA======Pregnancy Category B=====
Risk Summary
- There are no adequate and well controlled studies of LATUDA use in pregnant women. Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
- LATUDA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Human Data
- Safe use of LATUDA during pregnancy or lactation has not been established; therefore, use of LATUDA in pregnancy, in nursing mothers, or in women of childbearing potential requires that the benefits of treatment be weighed against the possible risks to mother and child.
Animal Data
- No adverse developmental effects were observed in a study in which pregnant rats were given lurasidone during the period of organogenesis and continuing through weaning at doses up to 10 mg/kg/day, which is approximately half of the maximum recommended human dose (MRHD) of 160 mg/day, based on mg/m2 body surface area.
- No teratogenic effects were seen in studies in which pregnant rats and rabbits were given lurasidone during the period of organogenesis at doses up to 25 and 50 mg/kg/day, respectively. These doses are 1.5- and 6-times, in rats and rabbits, respectively, the MRHD of 160 mg/day based on mg/m2 body surface area.
|useInPregnancyAUS=*There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lurasidone in women who are pregnant. |useInLaborDelivery=*There is no FDA guidance on use of Lurasidone during labor and delivery. |useInNursing=*LATUDA was excreted in milk of rats during lactation. It is not known whether LATUDA or its metabolites are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, considering the risk of drug discontinuation to the mother. |useInPed=*Safety and effectiveness in pediatric patients have not been established. |useInGeri=*Clinical studies with LATUDA did not include sufficient numbers of patients aged 65 and older to determine whether or not they respond differently from younger patients. In elderly patients with psychosis (65 to 85), LATUDA concentrations (20 mg/day) were similar to those in young subjects. It is unknown whether dose adjustment is necessary on the basis of age alone.
- Elderly patients with dementia-related psychosis treated with LATUDA are at an increased risk of death compared to placebo. LATUDA is not approved for the treatment of patients with dementia-related psychosis
|useInGender=*There is no FDA guidance on the use of Lurasidone with respect to specific gender populations. |useInRace=*There is no FDA guidance on the use of Lurasidone with respect to specific racial populations. |useInRenalImpair=*Dose adjustment is recommended in moderate (creatinine clearance: 30 to <50 mL/min) and severe renal impairment (creatinine clearance <30 mL/min) patients. The recommended starting dose is 20 mg per day. The dose in these patients should not exceed 80 mg per day. |useInHepaticImpair=*Dose adjustment is recommended in moderate (Child-Pugh Score = 7 to 9) and severe hepatic impairment (Child-Pugh Score = 10 to 15) patients. The recommended starting dose is 20 mg per day. The dose in moderate hepatic impairment patients should not exceed 80 mg per day and the dose in severe hepatic impairment patients should not exceed 40 mg/day |useInReproPotential=*here is no FDA guidance on the use of Lurasidone in women of reproductive potentials and males. |useInImmunocomp=*There is no FDA guidance one the use of Lurasidone in patients who are immunocompromised. |othersTitle=Other Patient Factors |useInOthers=*The effect of intrinsic patient factors on the pharmacokinetics of LATUDA is presented in Figure 3.
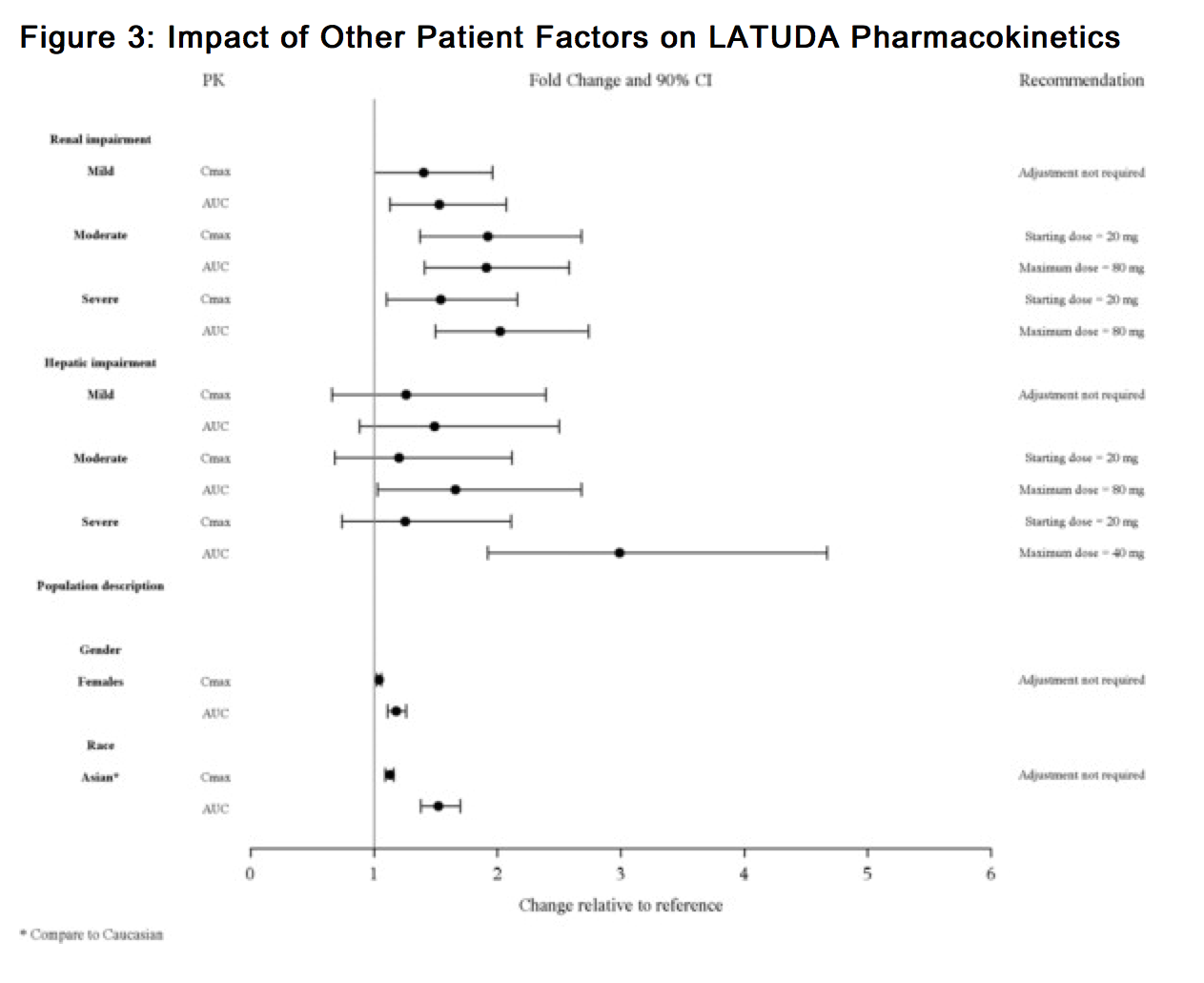
|administration=*ORAL
- LATUDA should be taken with food (at least 350 calories). Administration with food substantially increases the absorption of LATUDA. Administration with food increases the AUC approximately 2-fold and increases the Cmax approximately 3-fold. In the clinical studies, LATUDA was administered with food
|monitoring=*Monitoring for the emergence of suicidal thoughts and behavior, manic/hypomanic symptoms, irritability, agitation, or unusual changes in behavior.
- Educate patients and caregivers about the risk of metabolic changes and the need for specific monitoring.
- Clinical monitoring of weight is recommended.
- In management of overdosage Cardiovascular monitoring should commence immediately, including continuous electrocardiographic monitoring for possible arrhythmias.
|IVCompat=*There is limited information regarding IV Compatibility of Lurasidone in the drug l |overdose======Human Experience=====
- In premarketing clinical studies, accidental or intentional overdosage of LATUDA was identified in one patient who ingested an estimated 560 mg of LATUDA. This patient recovered without sequelae. This patient resumed LATUDA treatment for an additional two months.
Management of Overdosage
- Consult a Certified Poison Control Center for up-to-date guidance and advice. There is no specific antidote to LATUDA, therefore, appropriate supportive measures should be instituted and close medical supervision and monitoring should continue until the patient recovers. Consider the possibility of multiple-drug overdose.
- Cardiovascular monitoring should commence immediately, including continuous electrocardiographic monitoring for possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine carry a theoretical hazard of additive QT-prolonging effects when administered in patients with an acute overdose of LATUDA. Similarly, the alpha-blocking properties of bretylium might be additive to those of LATUDA, resulting in problematic hypotension.
- Hypotension and circulatory collapse should be treated with appropriate measures. Epinephrine and dopamine should not be used, or other sympathomimetics with beta-agonist activity, since beta stimulation may worsen hypotension in the setting of LATUDA-induced alpha blockade. In case of severe extrapyramidal symptoms, anticholinergic medication should be administered.
Gastric lavage (after intubation if patient is unconscious) and administration of activated charcoal together with a laxative should be considered.
- The possibility of obtundation, seizures, or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis.
|drugBox={{Drugbox2 | IUPAC_name = (3aR,4S,7R,7aS)-2-[((1R,2R)-2-{[4-(1,2-benzisothiazol-3-yl)-piperazin-1-yl]methyl}cyclohexyl)methyl]hexahydro-1H-4,7-methanisoindol-1,3-dione | image = Lurasidone_structure.png | width = 300 | image2 = Lurasidone3Dan.gif | width2 = 300
| tradename = Latuda | Drugs.com = Consumer Drug Information | MedlinePlus = a611016 | DailyMedID = 88a244d1-eddb-499c-bee2-e1f49056e78f | licence_US = Lurasidone | pregnancy_AU = B1 | pregnancy_US = B | pregnancy_category = | legal_AU = S4 | legal_CA = Rx-only | legal_US = Rx-only | legal_status = | routes_of_administration = Oral
| bioavailability = 9–19% (oral)[1]
| metabolism = Hepatic (CYP3A4-mediated)[1]
| elimination_half-life = 18 hours[1]
| excretion = Faecal (~80%), renal (~9%)[1]
| CAS_number = 367514-87-2
| ATC_prefix = N05
| ATC_suffix = AE05
| PubChem = 213046
| ChemSpiderID = 184739
| UNII_Ref =
| UNII = 22IC88528T
| ChEMBL = 1237021
| ChEBI_Ref =
| ChEBI = 70735
| synonyms = SM-13,496
| C = 28 | H = 36 | N = 4 | O = 2 | S = 1 | molecular_weight = 492.676 g/mol | smiles = O=C1N(C(=O)[C@H]3[C@@H]1[C@@H]2CC[C@H]3C2)C[C@@H]4CCCC[C@H]4CN7CCN(c6nsc5ccccc56)CC7 | StdInChI=1S/C28H36N4O2S/c33-27-24-18-9-10-19(15-18)25(24)28(34)32(27)17-21-6-2-1-5-20(21)16-30-11-13-31(14-12-30)26-22-7-3-4-8-23(22)35-29-26/h3-4,7-8,18-21,24-25H,1-2,5-6,9-17H2/t18-,19+,20-,21-,24+,25-/m0/s1 | StdInChIKey=PQXKDMSYBGKCJA-CVTJIBDQSA-N }} |mechAction=*The mechanism of action of LATUDA in the treatment of schizophrenia and bipolar depression is unknown. However, its efficacy in schizophrenia and bipolar depression could be mediated through a combination of central dopamine Type 2 (D2) and serotonin Type 2 (5HT2A) receptor antagonism. |structure=*LATUDA is an atypical antipsychotic belonging to the chemical class of benzisothiazol derivatives.
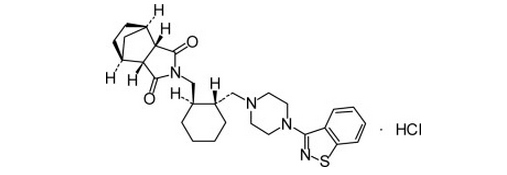
- Its chemical name is (3aR,4S,7R,7aS)-2-(1R,2R)-2-4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl cyclohexylmethylhexahydro-4,7-methano-2H-isoindole-1,3-dione hydrochloride. Its molecular formula is C28H36N4O2S·HCl and its molecular weight is 529.14.
- The chemical structure is:
- Lurasidone hydrochloride is a white to off-white powder. It is very slightly soluble in water, practically insoluble or insoluble in 0.1 N HCl, slightly soluble in ethanol, sparingly soluble in methanol, practically insoluble or insoluble in toluene and very slightly soluble in acetone.
- LATUDA tablets are intended for oral administration only. Each tablet contains 20 mg, 40 mg, 60 mg, 80 mg, or 120 mg of lurasidone hydrochloride.
- Inactive ingredients are mannitol, pregelatinized starch, croscarmellose sodium, hypromellose, magnesium stearate, Opadry® and carnauba wax. Additionally, the 80 mg tablet contains yellow ferric oxide and FD&C Blue No. 2 Aluminum Lake.
|PD=*LATUDA is an antagonist with high affinity binding at the dopamine D2 receptors (Ki=1 nM) and the 5-hydroxytryptamine (5-HT, serotonin) receptors 5-HT2A (Ki=0.5 nM) and 5-HT7 (Ki=0.5 nM) receptors. It also binds with moderate affinity to the human α2C adrenergic receptors (Ki=11 nM), is a partial agonist at serotonin 5-HT1A (Ki=6.4 nM) receptors, and is an antagonist at the α2A adrenergic receptors (Ki=41 nM). LATUDA exhibits little or no affinity for histamine H1 and muscarinic M1 receptors (IC50 > 1,000 nM).
ECG Changes
- The effects of LATUDA on the QTc interval were evaluated in a randomized, double-blind, multiple-dose, parallel-dedicated thorough QT study in 43 patients with schizophrenia or schizoaffective disorder, who were treated with LATUDA doses of 120 mg daily, 600 mg daily and completed the study. The maximum mean (upper 1-sided, 95% CI) increase in baseline-adjusted QTc intervals based on individual correction method (QTcI) was 7.5 (11.7) ms and 4.6 (9.5) ms, for the 120 mg and 600 mg dose groups respectively, observed at 2 to 4 hours after dosing. In this study, there was no apparent dose (exposure)-response relationship.
- In short-term, placebo-controlled studies in schizophrenia and bipolar depression, no post-baseline QT prolongations exceeding 500 msec were reported in patients treated with LATUDA or placebo.
|PK=*The activity of LATUDA is primarily due to the parent drug. The pharmacokinetics of LATUDA is dose-proportional within a total daily dose range of 20 mg to 160 mg. Steady-state concentrations of LATUDA are reached within 7 days of starting LATUDA.
- Following administration of 40 mg of LATUDA, the mean (%CV) elimination half-life was 18 (7) hours.
Absorption and Distribution
- LATUDA is absorbed and reaches peak serum concentrations in approximately 1-3 hours. It is estimated that 9-19% of an administered dose is absorbed. Following administration of 40 mg of LATUDA, the mean (%CV) apparent volume of distribution was 6173 (17.2) L. LATUDA is highly bound (~99%) to serum proteins.
- In a food effect study, LATUDA mean Cmax and AUC were about 3-times and 2-times, respectively, when administered with food compared to the levels observed under fasting conditions. LATUDA exposure was not affected as meal size was increased from 350 to 1000 calories and was independent of meal fat content [see Dosage and Administration (2.3)].
- In clinical studies, establishing the safety and efficacy of LATUDA, patients were instructed to take their daily dose with food.
Metabolism and Elimination
- LATUDA is metabolized mainly via CYP3A4. The major biotransformation pathways are oxidative N-dealkylation, hydroxylation of norbornane ring, and S-oxidation. LATUDA is metabolized into two active metabolites (ID-14283 and ID-14326) and two major non-active metabolites (ID-20219 and ID-20220). Based on in vitro studies, LATUDA is not a substrate of CYP1A1, CYP1A2, CYP2A6, CYP4A11, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP2E1 enzymes. Because LATUDA is not a substrate for CYP1A2, smoking is not expected to have an effect on the pharmacokinetics of LATUDA.
- Total excretion of radioactivity in urine and feces combined was approximately 89%, with about 80% recovered in feces and 9% recovered in urine, after a single dose of [14C]-labeled LATUDA.
- Following administration of 40 mg of LATUDA, the mean (%CV) apparent clearance was 3902 (18.0) mL/min.
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility====
Carcinogenesis
- LATUDA increased incidences of malignant mammary gland tumors and pituitary gland adenomas in female mice orally dosed with 30, 100, 300, or 650 mg/kg/day. The lowest dose produced plasma levels (AUC) approximately equal to those in humans receiving the MRHD of 160 mg/day. No increases in tumors were seen in male mice up to the highest dose tested, which produced plasma levels (AUC) 14-times those in humans receiving the MRHD.
- LATUDA increased the incidence of mammary gland carcinomas in females rats orally dosed at 12 and 36 mg/kg/day: the lowest dose; 3 mg/kg/day is the no-effect dose which produced plasma levels (AUC) 0.4-times those in humans receiving the MRHD. No increases in tumors were seen in male rats up to the highest dose tested, which produced plasma levels (AUC) 6-times those in humans receiving the MRHD.
- Proliferative and/or neoplastic changes in the mammary and pituitary glands of rodents have been observed following chronic administration of antipsychotic drugs and are considered to be prolactin-mediated. The relevance of this increased incidence of prolactin-mediated pituitary or mammary gland tumors in rodents to humans is unknown [see Warnings and Precautions (5.7)].
Mutagenesis
- LATUDA did not cause mutation or chromosomal aberration when tested in vitro and in vivo. LATUDA was negative in the Ames gene mutation test, the Chinese Hamster Lung (CHL) cells, and in the in vivo mouse bone marrow micronucleus test up to 2000 mg/kg (61 times the MRHD of 160 mg/day based on mg/m2 body surface area).
Impairment of Fertility
- Estrus cycle irregularities were seen in rats orally administered LATUDA at 1.5, 15 and 150 mg/kg/day for 15 consecutive days prior to mating, during the mating period, and through day 7 of gestation. The no-effect dose is 0.1 mg/kg which is approximately 0.006-times the MRHD of 160 mg/day based on body surface area. Fertility was reduced only at the highest dose, which was reversible after a 14-day drug-free period. The no-effect dose for reduced fertility was 15 mg/kg, which is approximately equal to the MRHD based on body surface area.
- LATUDA had no effect on fertility in male rats treated orally with LATUDA for 64 consecutive days prior to mating and during the mating period at doses up to 150 mg/kg/day (9-times the MRHD based on mg/m2 body surface area).
|clinicalStudies=====Schizophrenia====
- The efficacy of LATUDA for the treatment of schizophrenia was established in five short-term (6-week), placebo-controlled studies in adult patients (mean age of 38.4 years, range 18-72) who met DSM-IV criteria for schizophrenia. An active-control arm (olanzapine or quetiapine extended-release) was included in two studies to assess assay sensitivity.
- Several instruments were used for assessing psychiatric signs and symptoms in these studies:
- Positive and Negative Syndrome Scale (PANSS), is a multi-item inventory of general psychopathology used to evaluate the effects of drug treatment in schizophrenia. PANSS total scores may range from 30 to 210.
- Brief Psychiatric Rating Scale derived (BPRSd), derived from the PANSS, is a multi-item inventory primarily focusing on positive symptoms of schizophrenia, whereas the PANSS includes a wider range of positive, negative and other symptoms of schizophrenia. The BPRSd consists of 18 items rated on a scale of 1 (not present) to 7 (severe). BPRSd scores may range from 18 to 126.
- The Clinical Global Impression severity scale (CGI-S) is a clinician-rated scale that measures the subject's current illness state on a 1- to 7-point scale.
- The endpoint associated with each instrument is change from baseline in the total score to the end of week 6. These changes are then compared to placebo changes for the drug and control groups.
- The results of the studies follow:
- Study 1: In a 6-week, placebo-controlled trial (N=145) involving two fixed doses of LATUDA (40 or 120 mg/day), both doses of LATUDA at Endpoint were superior to placebo on the BPRSd total score, and the CGI-S.
- Study 2: In a 6-week, placebo-controlled trial (N=180) involving a fixed dose of LATUDA (80 mg/day), LATUDA at Endpoint was superior to placebo on the BPRSd total score, and the CGI-S.
- Study 3: In a 6-week, placebo- and active-controlled trial (N=473) involving two fixed doses of LATUDA (40 or 120 mg/day) and an active control (olanzapine), both LATUDA doses and the active control at Endpoint were superior to placebo on the PANSS total score, and the CGI-S.
- Study 4: In a 6-week, placebo-controlled trial (N=489) involving three fixed doses of LATUDA (40, 80 or 120 mg/day), only the 80 mg/day dose of LATUDA at Endpoint was superior to placebo on the PANSS total score, and the CGI-S.
- Study 5: In a 6-week, placebo- and active-controlled trial (N=482) involving two fixed doses of LATUDA (80 or 160 mg/day) and an active control (quetiapine extended-release), both LATUDA doses and the active control at Endpoint were superior to placebo on the PANSS total score, and the CGI-S.
- Thus, the efficacy of LATUDA at doses of 40, 80, 120 and 160 mg/day has been established (Table 24).
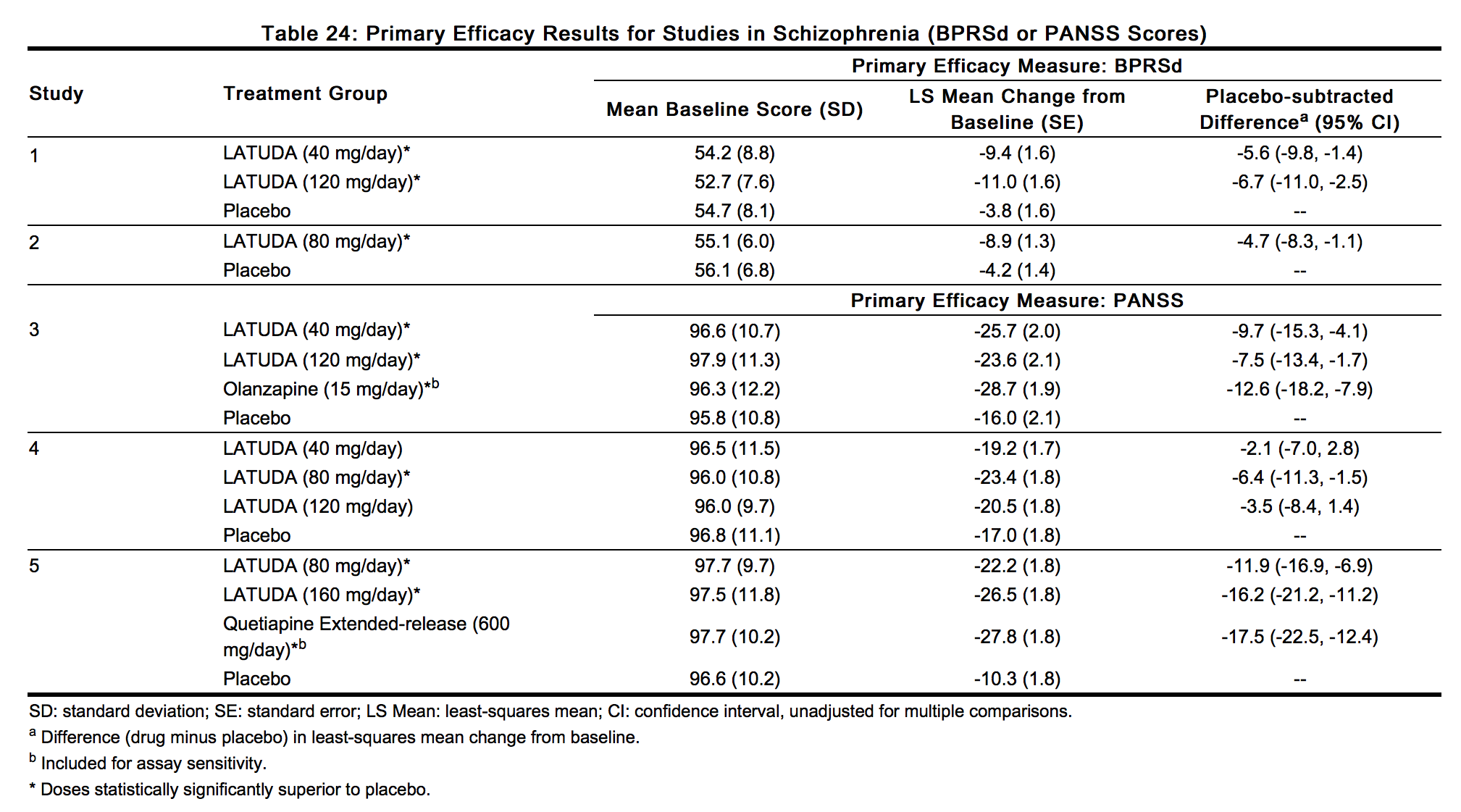
- Examination of population subgroups based on age (there were few patients over 65), gender and race did not reveal any clear evidence of differential responsiveness.
Depressive Episodes Associated with Bipolar I Disorder
Monotherapy
- The efficacy of LATUDA, as monotherapy, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adult patients (mean age of 41.5 years, range 18 to 74) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=485). Patients were randomized to one of two flexible-dose ranges of LATUDA (20 to 60 mg/day, or 80 to 120 mg/day) or placebo.
- The primary rating instrument used to assess depressive symptoms in this study was the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-rated scale with total scores ranging from 0 (no depressive features) to 60 (maximum score). The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the Clinical Global Impression-Bipolar-Severity of Illness scale (CGI-BP-S), a clinician-rated scale that measures the subject's current illness state on a 7-point scale, where a higher score is associated with greater illness severity.
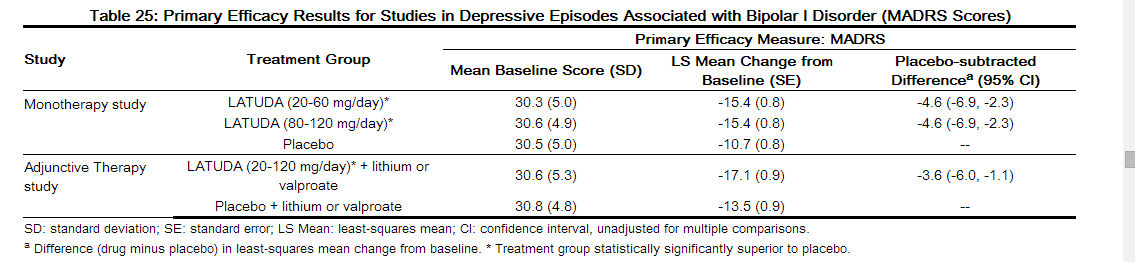
- For both dose groups, LATUDA was superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6. The primary efficacy results are provided in Table 25. The high dose range (80 to 120 mg per day) did not provide additional efficacy on average, compared to the low dose range (20 to 60 mg per day).
Adjunctive Therapy with Lithium or Valproate
- The efficacy of LATUDA, as an adjunctive therapy with lithium or valproate, was established in a 6-week, multicenter, randomized, double-blind, placebo-controlled study of adult patients (mean age of 41.7 years, range 18 to 72) who met DSM-IV-TR criteria for major depressive episodes associated with bipolar I disorder, with or without rapid cycling, and without psychotic features (N=340). Patients who remained symptomatic after treatment with lithium or valproate were randomized to flexibly dosed LATUDA 20 to 120 mg/day or placebo.
- The primary rating instrument used to assess depressive symptoms in this study was the MADRS. The primary endpoint was the change from baseline in MADRS score at Week 6. The key secondary instrument was the CGI-BP-S scale.
- LATUDA was superior to placebo in reduction of MADRS and CGI-BP-S scores at Week 6, as an adjunctive therapy with lithium or valproate (Table 25).
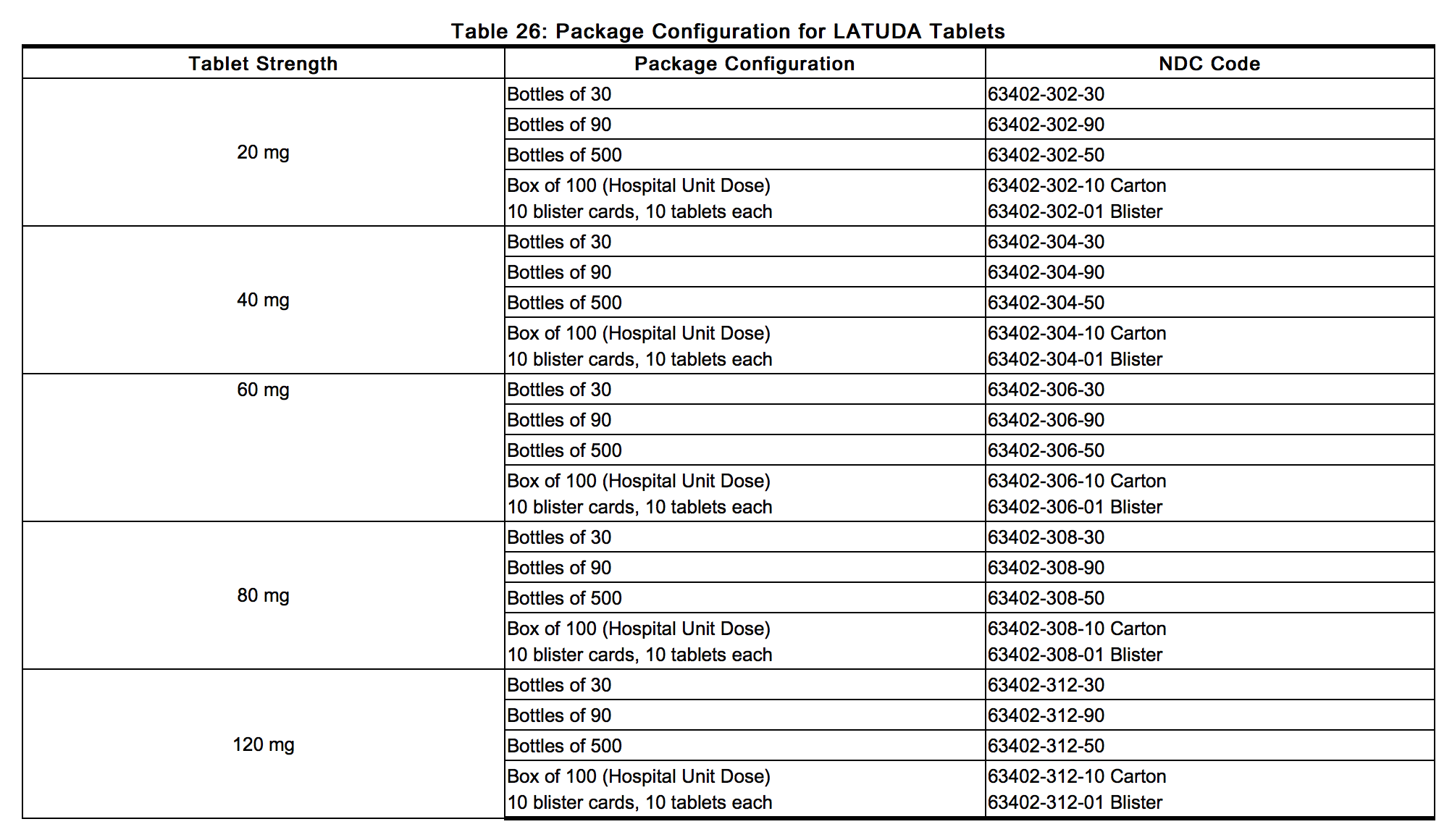
|howSupplied=*LATUDA tablets are white to off-white, round (20 mg or 40 mg), white to off-white, oblong (60 mg), pale green, oval (80 mg) or white to off-white, oval (120 mg) and identified with strength-specific one-sided debossing, “L20” (20 mg), “L40” (40 mg), “L80” (80 mg) or “L120” (120 mg). Tablets are supplied in the following strengths and package configurations (Table 26):
|storage=*Store LATUDA tablets at 25°C (77°F); excursions permitted to 15° - 30°C (59° - 86°F).
|fdaPatientInfo=

|alcohol=*Advise patients to avoid alcohol while taking LATUDA. |brandNames=* ®[2]
|lookAlike=* A® — B®[3]
|drugShortage= }} {{#subobject:
|Page Name=Lurasidone
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Lurasidone |Label Name=Lurasidone11.png
}}
{{#subobject:
|Label Page=Lurasidone |Label Name=Lurasidone11.png
}}
- ↑ 1.0 1.1 1.2 1.3 "PRODUCT INFORMATION LATUDA (lurasidone hydrochloride)" (PDF). TGA eBusiness Services. Therapeutic Goods Administration. 16 April 2014. Retrieved 1 May 2014.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)