Chagas disease pathophysiology: Difference between revisions
m (Bot: Removing from Primary care) |
|||
| (44 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{Chagas disease}} | {{Chagas disease}} | ||
{{CMG}}; {{AE}} {{YD}}, {{RT}} | |||
==Overview== | ==Overview== | ||
The most common mode of [[transmission]] of Chagas disease is vectorborne via exposure to [[feces]]/[[urine]] of infected triatomine insects. Nonetheless, other modes of [[transmission]], such as via [[blood transfusion]], [[organ transplant]], [[oral]] [[ingestion]], [[breast milk]], and vertical [[transmission]], are possible. The hallmark of Chagas disease is [[inflammation]]. Acutely following transmission, a state of [[parasitemia]], characterized by [[parasitic]] [[replication]] and [[Hosts|host]] [[immune]] responses, is responsible for the development of [[clinical]] manifestations. Following the [[acute]] phase, it is thought that Chagas disease causes a state of low-grade persistent [[inflammation]] that eventually results in the development of [[chronic]] disease, manifested by multisystem involvement. The [[pathogenesis]] of [[chronic]] Chagas disease is poorly understood, but is thought to be caused by either persistent [[parasites]] that were not eliminated by the host during the acute phase or development of [[autoimmune]] destructive processes. On [[gross pathology]], [[acute]] Chagas disease is characterized by [[inflammation]], whereas [[chronic]] disease often demonstrates tissue dilation and [[congestion]], along with evidence of [[fibrosis]] and wall thickening. On [[microscopic]] [[histopathological]] analysis, [[acute]] Chagas disease demonstrates [[mononuclear]] and [[lymphocytic]] infiltration in [[infected]] tissue, which chronically result in tissue denervation and fibrous scar formation. The [[histologic]] diagnosis of [[Cardiomyopathy|Chronic Chagas cardiomyopathy]] (CCM) consists of a diffuse and patchy [[chronic myocarditis]], [[interstitial]] [[mononuclear cell]] infiltrates, and [[myocardial]] fiber destruction with fibrotic replacement. Grossly enlarged hearts have been found in [[autopsy]] studies in subjects with end stage of Chagas disease. [[Left ventricular]] apical aneurysms are also frequently found on [[autopsy]]. | |||
== | ==Pathophysiology== | ||
===Transmission=== | |||
*Chagas disease usually has a vector-borne transmission. Triatomine insects, the Riduvid (kissing/assassin) bugs, suck blood from an infected individual and are subsequently infected themselves. | |||
*The insects carry the pathogen in their feces and urine. Human infection with ''T. cruzi'' occurs following exposure to feces/urine of infected insects. The pathogen typically enters the host either through a wound induced by the host's scratching following the insect bite or through the conjunctival mucus membranes. | |||
*Other modes of transmission include organ transplantation, blood transfusions, vertical transmission, breast milk, and oral transmission following ingestion of infected foods.<ref>Santos Ferreira C, Amato Neto V, Gakiya E, ''et al.'' "Microwave treatment of human milk to prevent transmission of Chagas disease." Rev Inst Med Trop São Paulo. 2003 Jan-Feb;45(1):41-2. PMID 12751321</ref><ref name="WHO">WHO. [http://www.who.int/tdr/diseases/chagas/ Chagas.] Accessed 24 September 2006.</ref><ref>da Silva Valente S, de Costa Valente V, Neto H. "Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon." Mem Inst Oswaldo Cruz 94 Suppl 1: 395-8. PMID 10677763</ref><ref>UK Health Protection Agency (HPA).[http://www.hpa.org.uk/cdr/archives/archive05/News/news1305.htm Chagas’ disease (American trypanosomiasis) in southern Brazil.] Accessed 24 September 2006.</ref> | |||
The | *The incubation period following transmission is typically 1-2 weeks following transmission. | ||
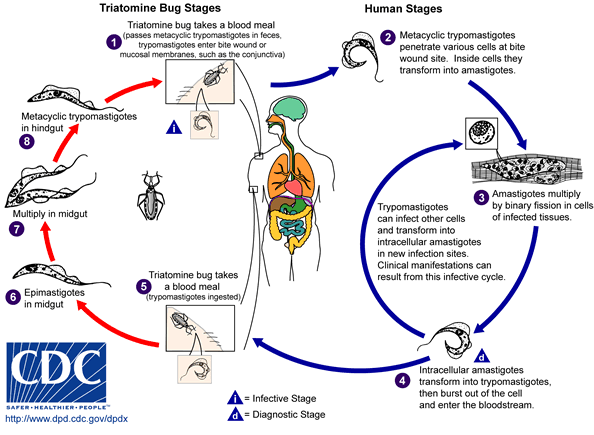
[[Image:Trypanosoma cruzi LifeCycle.gif|center||800px|thumb|Life cycle of [[Trypanosoma cruzi]]. - Source: https://www.cdc.gov/]] | |||
===Cellular Pathogenesis=== | |||
====Acute infection==== | |||
* Following transmission, trypomastigotes invade host cells via a unique transmigration process that involves bradykinin and CCL2 chemokine. | |||
*Unlike African [[trypanosomes]], the bloodstream trypomastigotes are unable to replicate. Instead, they differentiate into intracellular [[amastigote]]s intracellularly. | |||
[[ | * The amastigotes multiply by [[binary fission]] and re-transform into trypomastigotes, which are released again into the blood and lymphatic circulations. These newly formed trypomastigotes are then able to infect new host tissues (macrophages, fibroblasts, skeletal and cardiac myocytes, and endothelial cells), which often accounts for the high grade parasitemia and the multisystem complications associated with Chagas disease. | ||
* Inflammation is the hallmark of Chagas disease. Early host immune responses include the activation of B-cell and T-cell (CD4+ and CD8+) lymphocytes that result in the production of anti-trypanosoma antibodies and direct cytotoxicity. It is thought that host immune mechanisms simultaneously contribute to the elimination of the parasite and host tissue damage. The early response causes a state of systemic inflammation reaction that either subsides and resolves or persists as a low-grade silent inflammation and manifests as a chronic disease.<ref name="pmid24312535">{{cite journal| author=Coates BM, Sullivan DP, Makanji MY, Du NY, Olson CL, Muller WA et al.| title=Endothelial transmigration by Trypanosoma cruzi. | journal=PLoS One | year= 2013 | volume= 8 | issue= 12 | pages= e81187 | pmid=24312535 | doi=10.1371/journal.pone.0081187 | pmc=PMC3846899 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24312535 }} </ref><ref name="pmid1549177">{{cite journal| author=Tarleton RL, Koller BH, Latour A, Postan M| title=Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. | journal=Nature | year= 1992 | volume= 356 | issue= 6367 | pages= 338-40 | pmid=1549177 | doi=10.1038/356338a0 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1549177 }} </ref> | |||
=== | ====Chronic infection==== | ||
*The true mechanism that result in chronic manifestations of the disease is poorly understood, but it is thought that chronic Chagas disease may be caused by either pathogenic inflammatory responses against residual and persistent ''T. cruzi'' pathogens or autoimmune mechanisms.<ref name="pmid15275290">{{cite journal| author=Kalil J, Cunha-Neto E| title=Autoimmunity in chagas disease cardiomyopathy: Fulfilling the criteria at last? | journal=Parasitol Today | year= 1996 | volume= 12 | issue= 10 | pages= 396-9 | pmid=15275290 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15275290 }} </ref><ref name="pmid11334941">{{cite journal| author=Tarleton RL| title=Parasite persistence in the aetiology of Chagas disease. | journal=Int J Parasitol | year= 2001 | volume= 31 | issue= 5-6 | pages= 550-4 | pmid=11334941 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11334941 }} </ref><ref name="pmid10194457">{{cite journal| author=Kierszenbaum F| title=Chagas' disease and the autoimmunity hypothesis. | journal=Clin Microbiol Rev | year= 1999 | volume= 12 | issue= 2 | pages= 210-23 | pmid=10194457 | doi= | pmc=PMC88915 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10194457 }} </ref> | |||
*Host immune cells, namely mononuclear cells, play an important role in the chronic host tissue destruction. Other involved immune cells include activated neutrophils and eosinophils. | |||
*Chronic inflammation typically results in end-organ damage by inducing local and diffuse tissue necrosis and fibrosis. | |||
*Denervation is characteristic of Chagas disease, whereby host organs lose [[sympathetic nervous system|sympathethic]] and [[parasympathetic nervous system|parasympathetic]] nerve endings (neuronolysis) within the walls of infected tissue. The denervation process results in clinical manifestations (e.g. GI hypomotility).<ref name="pmid17148699">{{cite journal| author=Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA| title=Chagas disease. | journal=Postgrad Med J | year= 2006 | volume= 82 | issue= 974 | pages= 788-98 | pmid=17148699 | doi=10.1136/pgmj.2006.047357 | pmc=PMC2653922 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17148699 }} </ref> | |||
===Gross Pathology=== | |||
*On goss pathology, infected organs, such as the heart, the colon, lymph nodes, or the esophagus, demonstrate the following characteristic features<ref name="pmid17148699">{{cite journal| author=Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA| title=Chagas disease. | journal=Postgrad Med J | year= 2006 | volume= 82 | issue= 974 | pages= 788-98 | pmid=17148699 | doi=10.1136/pgmj.2006.047357 | pmc=PMC2653922 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17148699 }} </ref>: | |||
:*Expansion in size | |||
:*Dilation with flabby-appearing tissue | |||
:*Tissue congestion and engorgement | |||
:*Tissue effacement and [[aneurysm]] formation (e.g. left ventricle apical effacement in Chagas cardiomyopathy) typically followed by life-threatening thrombus formation | |||
:*Whitish "soldier's" patches (early) and diffuse fibrosis (late) suggestive of tissue fibrosis in chronic states | |||
===Microscopic Pathology=== | |||
Microscopic histopathological analysis of organs infected with Chagas disease often demonstrate the following characteristic features<ref name="pmid17148699">{{cite journal| author=Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA| title=Chagas disease. | journal=Postgrad Med J | year= 2006 | volume= 82 | issue= 974 | pages= 788-98 | pmid=17148699 | doi=10.1136/pgmj.2006.047357 | pmc=PMC2653922 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17148699 }} </ref>: | |||
====Acute Phase==== | |||
*[[Pseudocyst]] formation in muscle fibers and histiocytes | |||
*[[Mononuclear]] and [[lymphocyte|lymphocytic]] cellular infiltrate | |||
*Host cell lysis (eg. [myocyte]]s, [[neuron]]s, [[endothelial cell]]s, brain [[astrocytes]]) with or without any evidence of parasite presence (especially in acute phase) | |||
*Evidence of ''T. cruzi'' parasite in the [[cytoplasm]] of host cells, such as cardiac myocytes or neurons. The parasite typically appear to be adhering to cellular [[microfilament]]s. | |||
*Palisading mononuclear cells around meningeal blood vessels (in Virchow-Robin spaces) | |||
====Indeterminate Phase==== | |||
*Low-grade inflammation in host tissue | |||
====Chronic Phase==== | |||
*Evidence of mononuclear and lymphocytic cellular infiltrate and host cell lysis | |||
*Replacement of destroyed tissue by fibrous scars, which typically appear locally then become more diffuse | |||
===Chagas heart disease=== | |||
Chagas disease is not | The [[pathogenesis]] of Chagas cardiomyopathy is incompletely understood, it may involve several mechanisms (Hypothesis), including: | ||
* Parasite-dependent myocardial damage, | |||
* Immune-mediated myocardial injury (induced by the parasite itself and by self-antigens) | |||
*Microvascular and neurogenic disturbances. | |||
There are two phases for Chagas heart disease, acute and chronic. | |||
'''[[Acute Chagas disease]]:''' | |||
Organ damage can occur as a result of intense parasitaemia and tissue parasitism, with superimposed [[immune]]-inflammatory response to the parasite. Although any organ can harbour the parasites, experimental Trypanosoma cruzi infection has a typical predilection for the muscle system in the heart, oesophagus and colon, and for the central nervous system. An in vitro model using human cell lines in culture to observe T. cruzi passage through the vascular barrier showed that there is usually no disruption of the endothelial monolayer, as the parasite uses a special transmigration process that is facilitated by bradykinin and CCL2 chemokine. It is not fully clear though that such phenomenon also occurs in vivo. | |||
Histopathology: | |||
prominent inflammatory changes in the vicinity of ruptured infected cells in tissues from both right and left cardiac chambers | |||
Myocarditis is intense and diffuse with myocyte necrosis, interstitial oedema, vasculitis and capillary dilation, and mononuclear and polymorphonuclear infiltration | |||
The immunological reaction is thought to control the active parasite multiplication through various host innate mechanisms that play a role in detecting and controlling parasite tissue invasion - a powerful reaction involving CD4+ and CD8+ T-cells and B-cells activation that induces direct antitrypanosoma cytotoxicity, cytokine secretion and production of specific antibodies against the parasite. The neuronal depopulation of the Meissner and Auerbach plexuses that occurs in esophageal and colon tissues during the acute phase are a key factor in the pathogenesis of megaoesophagus and megacolon in the chronic phase. Direct damage of smooth muscle may also be a contributory factor, but this hypothesis has not been adequately explored in humans and animal models of Chagas disease. | |||
'''Chronic Chagas cardiomyopathy:''' | |||
The pathogenic mechanisms responsible for cardiac lesions developing during the chronic phase of Chagas disease are not completely understood. However, four mechanisms are believed to play a role, neurogenic disturbances, microvascular derangements, parasite-dependent damage, and immune-mediated tissue injury. The first two mechanisms probably play only an ancillary role in the development of the cardiac lesions and clinical complications observed in patients with chronic Chagas cardiomyopathy. By contrast, most investigators now believe that parasite persistence is a critical factor in causing inflammation and in initiating and progressing chronic myocarditis. This concept emphesizes on the notion that chronic Chagas disease is indeed an infectious entity, in which the parasite is not completely eliminated, despite the multiple and proteiform reactions developed by the host systems against it. | |||
[[Acute]] form of Chagas Disease: [[Myocarditis]] is infrequent, appearing in only 1-5% of patients whose having the [[acute]] phase of [[Chagas Disease]] (1-5 of every 10,000 infected subjects). | |||
[[Chronic]] form of Chagas Disease: The [[histologic]] [[diagnosis]] of [[Cardiomyopathy|Chronic Chagas cardiomyopathy]] (CCM) consists of a diffuse and patchy [[chronic myocarditis]], [[interstitial]] [[mononuclear cell]] infiltrates, and [[myocardial]] fiber destruction with fibrotic replacement. Grossly enlarged hearts have been found in [[autopsy]] studies in subjects with end stage of Chagas disease. [[Left ventricular]] [[apical]] [[aneurysms]] are also frequently found on [[autopsy]]. | |||
The degree of [[myocardial]] [[fibrosis]] increases progressively from the mildest to the most severe disease stages. Additionally, [[myocardial]] [[fibrosis]] correlates inversely with [[left ventricular ejection fraction]] and clinical status. | |||
[[Cardiac MRI]] may demonstrate [[myocardial]] involvement (hyperenhancement) among [[seropositive]] patients without [[clinical]] [[symptoms]] or [[left ventricular]] wall motion abnormalities. | |||
Across groups A-D, [[coronary angiography]] is usually normal or shows minimally obstructive disease. | |||
==Case Example: Heart Involvement in Chagas Disease == | |||
===Clinical Summary=== | |||
A 12-year-old boy, whose family had recently emigrated from Brazil, presented to the emergency room with a three-day history of malaise, fever, anorexia, and edema of the face and upper extremities. | |||
On physical examination the patient had generalized lymphadenopathy and hepatosplenomegaly. | |||
The patient was tachycardic and dysgenic with signs of congestive heart failure. | |||
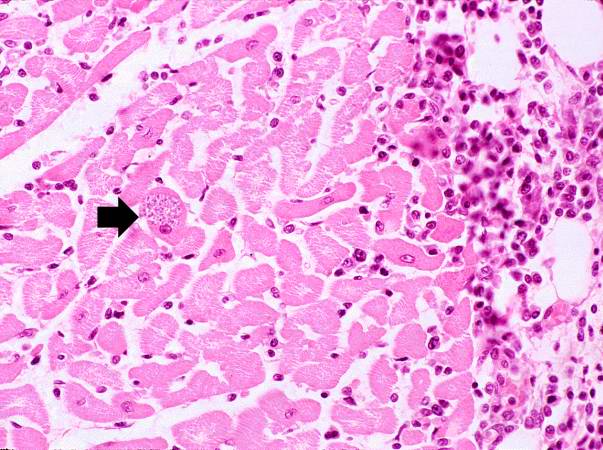
A cardiac biopsy was performed which revealed an active myocarditis with leishmanial forms of parasitic organisms within cardiac myocytes. | |||
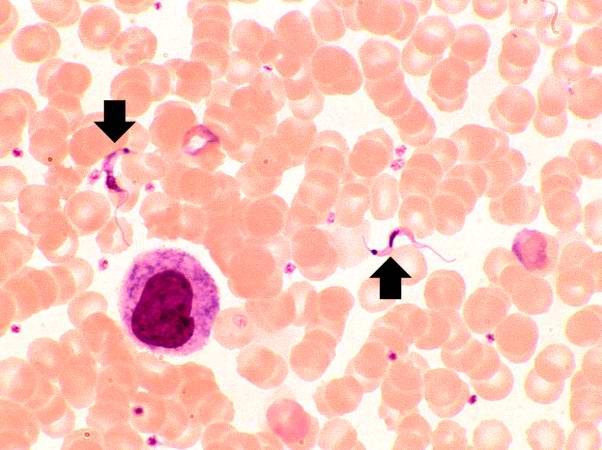
Close examination of peripheral blood smears revealed occasional circulating trypomastigotes. A complement fixation test for antibodies to Trypanosoma cruzi was strongly positive. | |||
==Gallery== | |||
<gallery> | |||
Image: Chagas03.jpeg| Life cycle of Trypanosoma cruzi, the causal agent of American Trypanosomiasis. <SMALL><SMALL>''[http://phil.cdc.gov/phil/home.asp From Public Health Image Library (PHIL).] ''<ref name=PHIL> {{Cite web | title = Public Health Image Library (PHIL) | url = http://phil.cdc.gov/phil/home.asp}}</ref></SMALL></SMALL> | |||
Image: Chagas09.jpeg| Trypanosoma cruzi in monkey heart. <SMALL><SMALL>''[http://phil.cdc.gov/phil/home.asp From Public Health Image Library (PHIL).] ''<ref name=PHIL> {{Cite web | title = Public Health Image Library (PHIL) | url = http://phil.cdc.gov/phil/home.asp}}</ref></SMALL></SMALL> | |||
</gallery> | |||
</gallery> | |||
[http://www.peir.net Images courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | |||
<div align="left"> | |||
<gallery heights="175" widths="175"> | |||
Image:Heart in Chagas disease 1.jpg|This peripheral blood smear from the patient shows two trypomastigotes of Trypanosoma cruzi. | |||
Image:Heart in Chagas disease 2.jpg|This peripheral blood smear from the patient shows a higher power view of a Trypanosoma cruzi trypomastigote. Note the prominent kinetoplast (arrow). | |||
</gallery> | |||
</div> | |||
<div align="left"> | |||
<gallery heights="175" widths="175"> | |||
Image:Heart in Chagas disease 3.jpg|This is a low-power photomicrograph of an H & E stained section from the heart biopsy of this patient. Note the organisms within a myocyte (arrow) and the adjacent inflammatory response. | |||
Image:Heart in Chagas disease 4.jpg|This is a higher-power photomicrograph of an H & E stained heart biopsy from this patient. Again, note the organisms within a myocyte (arrow) and the inflammatory response. | |||
</gallery> | |||
</div> | |||
<div align="left"> | |||
<gallery heights="175" widths="175"> | |||
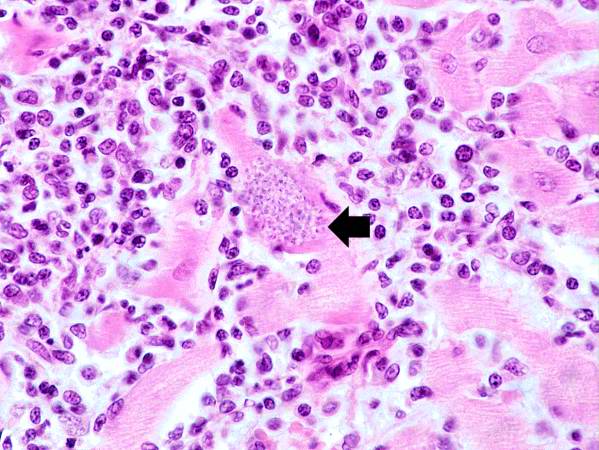
Image:Heart in Chagas disease 5.jpg|This is a higher-power photomicrograph of an H & E stained heart biopsy from this patient. At this magnification the organisms within a myocyte (arrows) and the adjacent inflammatory response are more clearly seen. The individual organisms within the myocyte are called amastigotes. | |||
Image:Heart in Chagas disease 6.jpg|This is a higher-power photomicrograph of an H & E stained heart biopsy from this patient. Note the T. cruzi amastigotes (arrows) within this longitudinal section of a myocyte. | |||
</gallery> | |||
</div> | |||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category:Disease]] | |||
[[Category:Up-To-Date]] | |||
[[Category:Neurology]] | |||
[[Category:Emergency medicine]] | |||
[[Category:Infectious disease]] | |||
[[Category:Gastroenterology]] | |||
[[Category:Cardiology]] | |||
Latest revision as of 20:53, 29 July 2020
|
Chagas disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Chagas disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Chagas disease pathophysiology |
|
Risk calculators and risk factors for Chagas disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yazan Daaboul, M.D., Raviteja Guddeti, M.B.B.S. [2]
Overview
The most common mode of transmission of Chagas disease is vectorborne via exposure to feces/urine of infected triatomine insects. Nonetheless, other modes of transmission, such as via blood transfusion, organ transplant, oral ingestion, breast milk, and vertical transmission, are possible. The hallmark of Chagas disease is inflammation. Acutely following transmission, a state of parasitemia, characterized by parasitic replication and host immune responses, is responsible for the development of clinical manifestations. Following the acute phase, it is thought that Chagas disease causes a state of low-grade persistent inflammation that eventually results in the development of chronic disease, manifested by multisystem involvement. The pathogenesis of chronic Chagas disease is poorly understood, but is thought to be caused by either persistent parasites that were not eliminated by the host during the acute phase or development of autoimmune destructive processes. On gross pathology, acute Chagas disease is characterized by inflammation, whereas chronic disease often demonstrates tissue dilation and congestion, along with evidence of fibrosis and wall thickening. On microscopic histopathological analysis, acute Chagas disease demonstrates mononuclear and lymphocytic infiltration in infected tissue, which chronically result in tissue denervation and fibrous scar formation. The histologic diagnosis of Chronic Chagas cardiomyopathy (CCM) consists of a diffuse and patchy chronic myocarditis, interstitial mononuclear cell infiltrates, and myocardial fiber destruction with fibrotic replacement. Grossly enlarged hearts have been found in autopsy studies in subjects with end stage of Chagas disease. Left ventricular apical aneurysms are also frequently found on autopsy.
Pathophysiology
Transmission
- Chagas disease usually has a vector-borne transmission. Triatomine insects, the Riduvid (kissing/assassin) bugs, suck blood from an infected individual and are subsequently infected themselves.
- The insects carry the pathogen in their feces and urine. Human infection with T. cruzi occurs following exposure to feces/urine of infected insects. The pathogen typically enters the host either through a wound induced by the host's scratching following the insect bite or through the conjunctival mucus membranes.
- Other modes of transmission include organ transplantation, blood transfusions, vertical transmission, breast milk, and oral transmission following ingestion of infected foods.[1][2][3][4]
- The incubation period following transmission is typically 1-2 weeks following transmission.
Cellular Pathogenesis
Acute infection
- Following transmission, trypomastigotes invade host cells via a unique transmigration process that involves bradykinin and CCL2 chemokine.
- Unlike African trypanosomes, the bloodstream trypomastigotes are unable to replicate. Instead, they differentiate into intracellular amastigotes intracellularly.
- The amastigotes multiply by binary fission and re-transform into trypomastigotes, which are released again into the blood and lymphatic circulations. These newly formed trypomastigotes are then able to infect new host tissues (macrophages, fibroblasts, skeletal and cardiac myocytes, and endothelial cells), which often accounts for the high grade parasitemia and the multisystem complications associated with Chagas disease.
- Inflammation is the hallmark of Chagas disease. Early host immune responses include the activation of B-cell and T-cell (CD4+ and CD8+) lymphocytes that result in the production of anti-trypanosoma antibodies and direct cytotoxicity. It is thought that host immune mechanisms simultaneously contribute to the elimination of the parasite and host tissue damage. The early response causes a state of systemic inflammation reaction that either subsides and resolves or persists as a low-grade silent inflammation and manifests as a chronic disease.[5][6]
Chronic infection
- The true mechanism that result in chronic manifestations of the disease is poorly understood, but it is thought that chronic Chagas disease may be caused by either pathogenic inflammatory responses against residual and persistent T. cruzi pathogens or autoimmune mechanisms.[7][8][9]
- Host immune cells, namely mononuclear cells, play an important role in the chronic host tissue destruction. Other involved immune cells include activated neutrophils and eosinophils.
- Chronic inflammation typically results in end-organ damage by inducing local and diffuse tissue necrosis and fibrosis.
- Denervation is characteristic of Chagas disease, whereby host organs lose sympathethic and parasympathetic nerve endings (neuronolysis) within the walls of infected tissue. The denervation process results in clinical manifestations (e.g. GI hypomotility).[10]
Gross Pathology
- On goss pathology, infected organs, such as the heart, the colon, lymph nodes, or the esophagus, demonstrate the following characteristic features[10]:
- Expansion in size
- Dilation with flabby-appearing tissue
- Tissue congestion and engorgement
- Tissue effacement and aneurysm formation (e.g. left ventricle apical effacement in Chagas cardiomyopathy) typically followed by life-threatening thrombus formation
- Whitish "soldier's" patches (early) and diffuse fibrosis (late) suggestive of tissue fibrosis in chronic states
Microscopic Pathology
Microscopic histopathological analysis of organs infected with Chagas disease often demonstrate the following characteristic features[10]:
Acute Phase
- Pseudocyst formation in muscle fibers and histiocytes
- Mononuclear and lymphocytic cellular infiltrate
- Host cell lysis (eg. [myocyte]]s, neurons, endothelial cells, brain astrocytes) with or without any evidence of parasite presence (especially in acute phase)
- Evidence of T. cruzi parasite in the cytoplasm of host cells, such as cardiac myocytes or neurons. The parasite typically appear to be adhering to cellular microfilaments.
- Palisading mononuclear cells around meningeal blood vessels (in Virchow-Robin spaces)
Indeterminate Phase
- Low-grade inflammation in host tissue
Chronic Phase
- Evidence of mononuclear and lymphocytic cellular infiltrate and host cell lysis
- Replacement of destroyed tissue by fibrous scars, which typically appear locally then become more diffuse
Chagas heart disease
The pathogenesis of Chagas cardiomyopathy is incompletely understood, it may involve several mechanisms (Hypothesis), including:
- Parasite-dependent myocardial damage,
- Immune-mediated myocardial injury (induced by the parasite itself and by self-antigens)
- Microvascular and neurogenic disturbances.
There are two phases for Chagas heart disease, acute and chronic.
Organ damage can occur as a result of intense parasitaemia and tissue parasitism, with superimposed immune-inflammatory response to the parasite. Although any organ can harbour the parasites, experimental Trypanosoma cruzi infection has a typical predilection for the muscle system in the heart, oesophagus and colon, and for the central nervous system. An in vitro model using human cell lines in culture to observe T. cruzi passage through the vascular barrier showed that there is usually no disruption of the endothelial monolayer, as the parasite uses a special transmigration process that is facilitated by bradykinin and CCL2 chemokine. It is not fully clear though that such phenomenon also occurs in vivo.
Histopathology:
prominent inflammatory changes in the vicinity of ruptured infected cells in tissues from both right and left cardiac chambers
Myocarditis is intense and diffuse with myocyte necrosis, interstitial oedema, vasculitis and capillary dilation, and mononuclear and polymorphonuclear infiltration
The immunological reaction is thought to control the active parasite multiplication through various host innate mechanisms that play a role in detecting and controlling parasite tissue invasion - a powerful reaction involving CD4+ and CD8+ T-cells and B-cells activation that induces direct antitrypanosoma cytotoxicity, cytokine secretion and production of specific antibodies against the parasite. The neuronal depopulation of the Meissner and Auerbach plexuses that occurs in esophageal and colon tissues during the acute phase are a key factor in the pathogenesis of megaoesophagus and megacolon in the chronic phase. Direct damage of smooth muscle may also be a contributory factor, but this hypothesis has not been adequately explored in humans and animal models of Chagas disease.
Chronic Chagas cardiomyopathy:
The pathogenic mechanisms responsible for cardiac lesions developing during the chronic phase of Chagas disease are not completely understood. However, four mechanisms are believed to play a role, neurogenic disturbances, microvascular derangements, parasite-dependent damage, and immune-mediated tissue injury. The first two mechanisms probably play only an ancillary role in the development of the cardiac lesions and clinical complications observed in patients with chronic Chagas cardiomyopathy. By contrast, most investigators now believe that parasite persistence is a critical factor in causing inflammation and in initiating and progressing chronic myocarditis. This concept emphesizes on the notion that chronic Chagas disease is indeed an infectious entity, in which the parasite is not completely eliminated, despite the multiple and proteiform reactions developed by the host systems against it.
Acute form of Chagas Disease: Myocarditis is infrequent, appearing in only 1-5% of patients whose having the acute phase of Chagas Disease (1-5 of every 10,000 infected subjects).
Chronic form of Chagas Disease: The histologic diagnosis of Chronic Chagas cardiomyopathy (CCM) consists of a diffuse and patchy chronic myocarditis, interstitial mononuclear cell infiltrates, and myocardial fiber destruction with fibrotic replacement. Grossly enlarged hearts have been found in autopsy studies in subjects with end stage of Chagas disease. Left ventricular apical aneurysms are also frequently found on autopsy.
The degree of myocardial fibrosis increases progressively from the mildest to the most severe disease stages. Additionally, myocardial fibrosis correlates inversely with left ventricular ejection fraction and clinical status.
Cardiac MRI may demonstrate myocardial involvement (hyperenhancement) among seropositive patients without clinical symptoms or left ventricular wall motion abnormalities.
Across groups A-D, coronary angiography is usually normal or shows minimally obstructive disease.
Case Example: Heart Involvement in Chagas Disease
Clinical Summary
A 12-year-old boy, whose family had recently emigrated from Brazil, presented to the emergency room with a three-day history of malaise, fever, anorexia, and edema of the face and upper extremities.
On physical examination the patient had generalized lymphadenopathy and hepatosplenomegaly.
The patient was tachycardic and dysgenic with signs of congestive heart failure.
A cardiac biopsy was performed which revealed an active myocarditis with leishmanial forms of parasitic organisms within cardiac myocytes.
Close examination of peripheral blood smears revealed occasional circulating trypomastigotes. A complement fixation test for antibodies to Trypanosoma cruzi was strongly positive.
Gallery
-
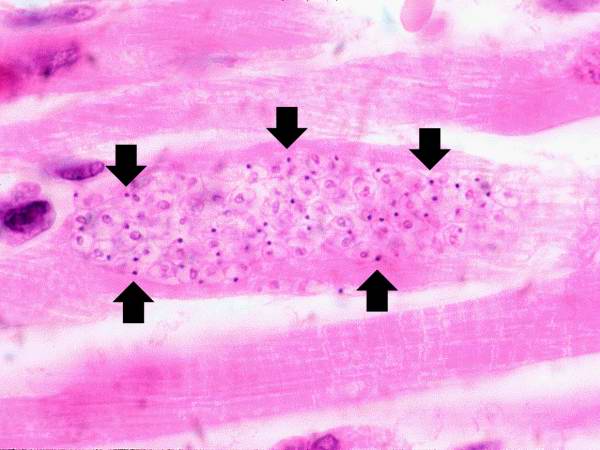
Life cycle of Trypanosoma cruzi, the causal agent of American Trypanosomiasis. From Public Health Image Library (PHIL). [11]
-
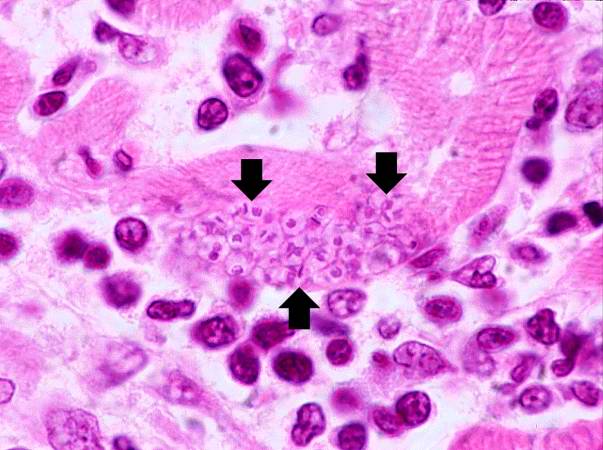
Trypanosoma cruzi in monkey heart. From Public Health Image Library (PHIL). [11]
</gallery>
-
This peripheral blood smear from the patient shows two trypomastigotes of Trypanosoma cruzi.
-
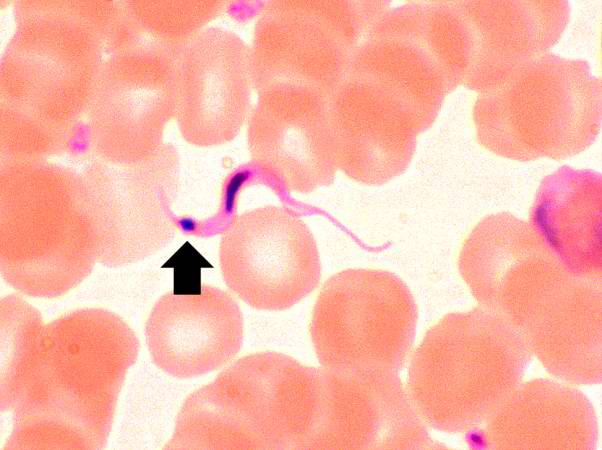
This peripheral blood smear from the patient shows a higher power view of a Trypanosoma cruzi trypomastigote. Note the prominent kinetoplast (arrow).
-
This is a low-power photomicrograph of an H & E stained section from the heart biopsy of this patient. Note the organisms within a myocyte (arrow) and the adjacent inflammatory response.
-
This is a higher-power photomicrograph of an H & E stained heart biopsy from this patient. Again, note the organisms within a myocyte (arrow) and the inflammatory response.
-
This is a higher-power photomicrograph of an H & E stained heart biopsy from this patient. At this magnification the organisms within a myocyte (arrows) and the adjacent inflammatory response are more clearly seen. The individual organisms within the myocyte are called amastigotes.
-
This is a higher-power photomicrograph of an H & E stained heart biopsy from this patient. Note the T. cruzi amastigotes (arrows) within this longitudinal section of a myocyte.
References
- ↑ Santos Ferreira C, Amato Neto V, Gakiya E, et al. "Microwave treatment of human milk to prevent transmission of Chagas disease." Rev Inst Med Trop São Paulo. 2003 Jan-Feb;45(1):41-2. PMID 12751321
- ↑ WHO. Chagas. Accessed 24 September 2006.
- ↑ da Silva Valente S, de Costa Valente V, Neto H. "Considerations on the epidemiology and transmission of Chagas disease in the Brazilian Amazon." Mem Inst Oswaldo Cruz 94 Suppl 1: 395-8. PMID 10677763
- ↑ UK Health Protection Agency (HPA).Chagas’ disease (American trypanosomiasis) in southern Brazil. Accessed 24 September 2006.
- ↑ Coates BM, Sullivan DP, Makanji MY, Du NY, Olson CL, Muller WA; et al. (2013). "Endothelial transmigration by Trypanosoma cruzi". PLoS One. 8 (12): e81187. doi:10.1371/journal.pone.0081187. PMC 3846899. PMID 24312535.
- ↑ Tarleton RL, Koller BH, Latour A, Postan M (1992). "Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection". Nature. 356 (6367): 338–40. doi:10.1038/356338a0. PMID 1549177.
- ↑ Kalil J, Cunha-Neto E (1996). "Autoimmunity in chagas disease cardiomyopathy: Fulfilling the criteria at last?". Parasitol Today. 12 (10): 396–9. PMID 15275290.
- ↑ Tarleton RL (2001). "Parasite persistence in the aetiology of Chagas disease". Int J Parasitol. 31 (5–6): 550–4. PMID 11334941.
- ↑ Kierszenbaum F (1999). "Chagas' disease and the autoimmunity hypothesis". Clin Microbiol Rev. 12 (2): 210–23. PMC 88915. PMID 10194457.
- ↑ 10.0 10.1 10.2 Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA (2006). "Chagas disease". Postgrad Med J. 82 (974): 788–98. doi:10.1136/pgmj.2006.047357. PMC 2653922. PMID 17148699.
- ↑ 11.0 11.1 "Public Health Image Library (PHIL)".
![Life cycle of Trypanosoma cruzi, the causal agent of American Trypanosomiasis. From Public Health Image Library (PHIL). [11]](/images/9/9e/Chagas03.jpeg)
![Trypanosoma cruzi in monkey heart. From Public Health Image Library (PHIL). [11]](/images/9/9a/Chagas09.jpeg)