Gastrointestinal stromal tumor pathophysiology: Difference between revisions
Akshun Kalia (talk | contribs) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
==Overview== | ==Overview== | ||
Gastrointestinal stromal tumors (GISTs) are rare but the most common [[mesenchymal]] (nonepithelial) [[tumors]] of the gastrointestinal tract. GISTs are derived from the [[interstitial cells of Cajal]] or | Gastrointestinal stromal tumors (GISTs) are rare but the most common [[mesenchymal]] (nonepithelial) [[tumors]] of the [[gastrointestinal tract]]. GISTs are derived from the [[interstitial cells of Cajal]] or undifferen2tiated [[precursor]] cells that finally develop into [[interstitial cells of Cajal]]. GIST [[Tumor|tumors]] can either be [[benign]] [[Tumor|tumors]] or massive [[malignant]] [[Tumor|tumors]] with widespread [[metastasis]]. They can occur in any part of the [[gastrointestinal tract]] with the most common location as [[stomach]]. GIST ([[tumors]]) can grow as an endophytic or exophytic [[Lesion|lesions]]. [[Genes]] involved in the [[pathogenesis]] of gastrointestinal stromal tumors include [[mutations]] in c-Kit [[gene]] and PDGFRA ([[platelet]] derived [[growth factor]] receptor-alpha) [[gene]]. Both Kit [[gene]] and PDGFRA are [[tyrosine kinase]] [[Receptor (biochemistry)|receptors]] and control [[cell proliferation]]. [[Mutation]] in c-Kit [[gene]] and PDGFRA leads to [[inhibition]] of [[apoptosis]] and uncontrolled [[cell proliferation]]. In some rare cases where the [[patient]] do not [[Exhibitionism|exhibit]] the typical [[mutation]] in c-Kit and PDGFRA, [[mutation|mutations]] in [[succinate dehydrogenase]] ([[SDH]]) have been reported. Conditions associated with GIST include [[urticaria pigmentosa]], [[neurofibromatosis type 1]], and Carney-Stratakis [[syndrome]]. On [[gross pathology]], GISTs have a rounded appearance with areas of [[hemorrhage]]. On [[microscopic]] [[histopathological]] [[analysis]], GISTs are [[cellular]] [[tumor|tumors]] arising from [[muscularis]] propria and composed of [[spindle cells]] (70%), [[Epithelioid cell|epithelioid cells]] (20%) or either one of them. | ||
==Pathophysiology== | ==Pathophysiology== | ||
* Gastrointestinal stromal tumors (GISTs) are rare but the most common [[mesenchymal]] (nonepithelial) [[tumors]] of the gastrointestinal tract.<ref name="pmid11213830">{{cite journal |vauthors=Miettinen M, Lasota J |title=Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis |journal=Virchows Arch. |volume=438 |issue=1 |pages=1–12 |year=2001 |pmid=11213830 |doi= |url=}}</ref> | * Gastrointestinal stromal tumors (GISTs) are rare but the most common [[mesenchymal]] (nonepithelial) [[tumors]] of the [[gastrointestinal tract]].<ref name="pmid11213830">{{cite journal |vauthors=Miettinen M, Lasota J |title=Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis |journal=Virchows Arch. |volume=438 |issue=1 |pages=1–12 |year=2001 |pmid=11213830 |doi= |url=}}</ref> | ||
* GIST tumors can either be [[benign]] tumors or massive [[malignant]] tumors with widespread [[metastasis]]. | * GIST tumors can either be [[benign]] [[Tumor|tumors]] or massive [[malignant]] [[Tumor|tumors]] with widespread [[metastasis]]. | ||
* Earlier GIST were thought to arise from the [[submucosal]] or [[smooth muscle cells]] of the [[Gastrointestinal tract|GI tract]]. However recent research have proved that GISTs are derived from the [[Interstitial cells of Cajal|interstitial cells of Caja]]<nowiki/>l or undifferentiated [[Precursors|precursor]] cells that finally develop into [[interstitial cells of Cajal]]. | * Earlier GIST were thought to arise from the [[submucosal]] or [[smooth muscle cells]] of the [[Gastrointestinal tract|GI tract]]. However recent research have proved that GISTs are derived from the [[Interstitial cells of Cajal|interstitial cells of Caja]]<nowiki/>l or undifferentiated [[Precursors|precursor]] cells that finally develop into [[interstitial cells of Cajal]].<ref name="miettinen">{{cite journal |author=Miettinen M, Lasota J |title=Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis |journal=Arch Pathol Lab Med |volume=130 |issue=10 |pages=1466-78 |year=2006 |id=PMID 17090188}}</ref><ref name="pmid9588894">{{cite journal |vauthors=Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM |title=Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal |journal=Am. J. Pathol. |volume=152 |issue=5 |pages=1259–69 |year=1998 |pmid=9588894 |pmc=1858579 |doi= |url=}}</ref> | ||
** [[Interstitial cells of Cajal]] (ICC) are a normal part of [[myenteric plexus]] and the [[autonomic nervous system]] of the [[intestine]]. | ** [[Interstitial cells of Cajal]] (ICC) are a normal part of [[myenteric plexus]] and the [[autonomic nervous system]] of the [[intestine]]. | ||
** [[Interstitial cells of Cajal]] serve as a [[pacemaker]] of [[intestine]] and controls intestinal motility. | ** [[Interstitial cells of Cajal]] serve as a [[pacemaker]] of [[intestine]] and controls [[intestinal]] motility. | ||
** [[Molecular]] analysis has shown that GISTs arising from the [[interstitial cells of Cajal]], stain positive for [[CD117]] (c-KIT) in 90% cases and CD34 in 70% of cases. Around 5% of the cases are positive for PDGFRA. The rest of the cases are defined as wild type (negative for both CD117 and PDGFRA). | ** [[Molecular]] [[analysis]] has shown that GISTs arising from the [[interstitial cells of Cajal]], stain positive for [[CD117]] (c-KIT) in 90% cases and [[CD34]] in 70% of cases. Around 5% of the cases are positive for PDGFRA. The rest of the cases are defined as wild type (negative for both [[CD117]] and PDGFRA). | ||
*** [[CD117]] is encoded by the ''KIT ''gene. Other names for [[CD117]] include proto-oncogene c-Kit and tyrosine kinase receptor Kit. | *** [[CD117]] is encoded by the ''KIT ''[[gene]]. Other names for [[CD117]] include [[Proto-oncogene protein C-kit|proto-oncogene c-Kit]] and [[tyrosine kinase]] [[Receptor (biochemistry)|receptor]] Kit. | ||
*** [[CD34]] is the myeloid progenitor cell antigen and also known as hematopoietic progenitor cell antigen CD34. | *** [[CD34]] is the [[myeloid]] [[progenitor cell]] [[antigen]] and also known as [[Hematopoiesis|hematopoietic]] [[progenitor]] [[Cell (biology)|cell]] [[antigen]] [[CD34]]. | ||
*** PDGFRA is platelet derived growth factor receptor-alpha and is a [[tyrosine kinase]] receptor. | *** PDGFRA is [[platelet]] derived [[growth factor]] [[Receptor (biochemistry)|receptor]]-alpha and is a [[tyrosine kinase]] [[Receptor (biochemistry)|receptor]]. | ||
* GIST can occur in any part of the [[Gastrointestinal tract|gastrointestinal tract.]] Thus, GIST vary considerably in their presentation and clinical course, ranging from being [[asymptomatic]] to presenting with severe signs and symptoms of [[bleeding]], [[abdominal pain]] and [[perforation]]. | * GIST can occur in any part of the [[Gastrointestinal tract|gastrointestinal tract.]] Thus, GIST vary considerably in their presentation and [[clinical]] course, ranging from being [[asymptomatic]] to presenting with severe signs and [[Symptom|symptoms]] of [[bleeding]], [[abdominal pain]] and [[perforation]].<ref name="pmid10824931">{{cite journal |vauthors=Reith JD, Goldblum JR, Lyles RH, Weiss SW |title=Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome |journal=Mod. Pathol. |volume=13 |issue=5 |pages=577–85 |year=2000 |pmid=10824931 |doi=10.1038/modpathol.3880099 |url=}}</ref> | ||
** The most common location for GIST is the [[stomach]] with the second most common location as the [[small intestine]]. | ** The most common location for GIST is the [[stomach]] with the second most common location as the [[small intestine]]. | ||
** Less frequent sites of occurrence include the [[colon]], [[rectum]] and [[esophagus]]. | ** Less frequent sites of occurrence include the [[colon]], [[rectum]] and [[esophagus]]. | ||
** Rare sites include [[pancreas]], [[peritoneum]], [[omentum]], or [[mesentery]]. | ** Rare sites include [[pancreas]], [[peritoneum]], [[omentum]], or [[mesentery]]. | ||
*GIST (tumors) can grow as an endophytic or exophytic lesions. | *GIST ([[Tumor|tumors]]) can grow as an endophytic or exophytic [[Lesion|lesions]]. | ||
**Endophytic lesions are [[benign]] linear lesions that grow along the [[lumen]] of the affected organ. | **Endophytic [[Lesion|lesions]] are [[benign]] linear [[Lesion|lesions]] that grow along the [[lumen]] of the affected [[Organ (anatomy)|organ]]. | ||
**Exophytic lesions can present as a protruding outgrowth outside the [[lumen]] of the [[Gastrointestinal tract|GI tract]]. | **Exophytic [[Lesion|lesions]] can present as a protruding outgrowth outside the [[lumen]] of the [[Gastrointestinal tract|GI tract]]. | ||
*GIST have a variable [[malignant]] potential.<ref name="pmid22153892">{{cite journal |vauthors=Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P |title=Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts |journal=Lancet Oncol. |volume=13 |issue=3 |pages=265–74 |year=2012 |pmid=22153892 |doi=10.1016/S1470-2045(11)70299-6 |url=}}</ref> | *GIST have a variable [[malignant]] potential.<ref name="pmid22153892">{{cite journal |vauthors=Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P |title=Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts |journal=Lancet Oncol. |volume=13 |issue=3 |pages=265–74 |year=2012 |pmid=22153892 |doi=10.1016/S1470-2045(11)70299-6 |url=}}</ref> | ||
**About 40% of GISTs that are localized at initial diagnosis give rise to [[metastasis]]. | **About 40% of GISTs that are localized at initial [[diagnosis]] give rise to [[metastasis]]. | ||
**Of all GIST, 10%-20% present with distant [[metastasis]] with the liver being the most frequent site of [[metastasis]].<ref name="pmid19620548">{{cite journal |vauthors=Woodall CE, Brock GN, Fan J, Byam JA, Scoggins CR, McMasters KM, Martin RC |title=An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system |journal=Arch Surg |volume=144 |issue=7 |pages=670–8 |year=2009 |pmid=19620548 |doi=10.1001/archsurg.2009.108 |url=}}</ref><ref name="pmid21953054">{{cite journal |vauthors=Emile JF, Brahimi S, Coindre JM, Bringuier PP, Monges G, Samb P, Doucet L, Hostein I, Landi B, Buisine MP, Neuville A, Bouché O, Cervera P, Pretet JL, Tisserand J, Gauthier A, Le Cesne A, Sabourin JC, Scoazec JY, Bonvalot S, Corless CL, Heinrich MC, Blay JY, Aegerter P |title=Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs |journal=Med. Oncol. |volume=29 |issue=3 |pages=1765–72 |year=2012 |pmid=21953054 |doi=10.1007/s12032-011-0074-y |url=}}</ref> | **Of all GIST, 10%-20% present with distant [[metastasis]] with the liver being the most frequent site of [[metastasis]].<ref name="pmid19620548">{{cite journal |vauthors=Woodall CE, Brock GN, Fan J, Byam JA, Scoggins CR, McMasters KM, Martin RC |title=An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system |journal=Arch Surg |volume=144 |issue=7 |pages=670–8 |year=2009 |pmid=19620548 |doi=10.1001/archsurg.2009.108 |url=}}</ref><ref name="pmid21953054">{{cite journal |vauthors=Emile JF, Brahimi S, Coindre JM, Bringuier PP, Monges G, Samb P, Doucet L, Hostein I, Landi B, Buisine MP, Neuville A, Bouché O, Cervera P, Pretet JL, Tisserand J, Gauthier A, Le Cesne A, Sabourin JC, Scoazec JY, Bonvalot S, Corless CL, Heinrich MC, Blay JY, Aegerter P |title=Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs |journal=Med. Oncol. |volume=29 |issue=3 |pages=1765–72 |year=2012 |pmid=21953054 |doi=10.1007/s12032-011-0074-y |url=}}</ref> | ||
**Other common sites of metastasis include the bone, peritoneum, retroperitoneum, lung, and pleura. | **Other common sites of [[metastasis]] include the [[bone]], [[peritoneum]], [[retroperitoneum]], [[lung]], and [[pleura]]. | ||
==Genetics== | ==Genetics== | ||
[[Genes]] involved in the [[pathogenesis]] of gastrointestinal stromal tumors include [[mutations]] in c-Kit gene and PDGFRA (platelet derived growth factor receptor-alpha) gene. In some rare cases where the patient do not exhibit the typical c-Kit and PDGFRA mutation, mutation in [[succinate dehydrogenase]] (SDH) have been reported. Rare [[genes]] involved include ''[[BRAF]]'' kinase, and [[protein kinase C]]. The majority of GISTs are sporadic in origin. <ref name="pmid14645423">{{cite journal |vauthors=Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA |title=Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor |journal=J. Clin. Oncol. |volume=21 |issue=23 |pages=4342–9 |year=2003 |pmid=14645423 |doi=10.1200/JCO.2003.04.190 |url=}}</ref><ref name="pmid9438854">{{cite journal |vauthors=Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y |title=Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors |journal=Science |volume=279 |issue=5350 |pages=577–80 |year=1998 |pmid=9438854 |doi= |url=}}</ref><ref name="DuensingMedeiros2004">{{cite journal|last1=Duensing|first1=Anette|last2=Medeiros|first2=Fabiola|last3=McConarty|first3=Bryna|last4=Joseph|first4=Nora E|last5=Panigrahy|first5=Dipak|last6=Singer|first6=Samuel|last7=Fletcher|first7=Christopher DM|last8=Demetri|first8=George D|last9=Fletcher|first9=Jonathan A|title=Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs)|journal=Oncogene|volume=23|issue=22|year=2004|pages=3999–4006|issn=0950-9232|doi=10.1038/sj.onc.1207525}}</ref><ref name="LuxRubin2000">{{cite journal|last1=Lux|first1=Marcia L.|last2=Rubin|first2=Brian P.|last3=Biase|first3=Tara L.|last4=Chen|first4=Chang-Jie|last5=Maclure|first5=Timothy|last6=Demetri|first6=George|last7=Xiao|first7=Sheng|last8=Singer|first8=Samuel|last9=Fletcher|first9=Christopher D.M.|last10=Fletcher|first10=Jonathan A.|title=KIT Extracellular and Kinase Domain Mutations in Gastrointestinal Stromal Tumors|journal=The American Journal of Pathology|volume=156|issue=3|year=2000|pages=791–795|issn=00029440|doi=10.1016/S0002-9440(10)64946-2}}</ref> | [[Genes]] involved in the [[pathogenesis]] of gastrointestinal stromal tumors include [[mutations]] in c-Kit [[gene]] and PDGFRA ([[platelet]] derived growth factor [[Receptor (biochemistry)|receptor]]-alpha) [[gene]]. In some rare cases where the [[patient]] do not [[Exhibitionism|exhibit]] the typical c-Kit and PDGFRA [[mutation]], mutation in [[succinate dehydrogenase]] (SDH) have been reported. Rare [[genes]] involved include ''[[BRAF]]'' kinase, and [[protein kinase C]]. The majority of GISTs are sporadic in origin. <ref name="pmid14645423">{{cite journal |vauthors=Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA |title=Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor |journal=J. Clin. Oncol. |volume=21 |issue=23 |pages=4342–9 |year=2003 |pmid=14645423 |doi=10.1200/JCO.2003.04.190 |url=}}</ref><ref name="pmid9438854">{{cite journal |vauthors=Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y |title=Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors |journal=Science |volume=279 |issue=5350 |pages=577–80 |year=1998 |pmid=9438854 |doi= |url=}}</ref><ref name="DuensingMedeiros2004">{{cite journal|last1=Duensing|first1=Anette|last2=Medeiros|first2=Fabiola|last3=McConarty|first3=Bryna|last4=Joseph|first4=Nora E|last5=Panigrahy|first5=Dipak|last6=Singer|first6=Samuel|last7=Fletcher|first7=Christopher DM|last8=Demetri|first8=George D|last9=Fletcher|first9=Jonathan A|title=Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs)|journal=Oncogene|volume=23|issue=22|year=2004|pages=3999–4006|issn=0950-9232|doi=10.1038/sj.onc.1207525}}</ref><ref name="LuxRubin2000">{{cite journal|last1=Lux|first1=Marcia L.|last2=Rubin|first2=Brian P.|last3=Biase|first3=Tara L.|last4=Chen|first4=Chang-Jie|last5=Maclure|first5=Timothy|last6=Demetri|first6=George|last7=Xiao|first7=Sheng|last8=Singer|first8=Samuel|last9=Fletcher|first9=Christopher D.M.|last10=Fletcher|first10=Jonathan A.|title=KIT Extracellular and Kinase Domain Mutations in Gastrointestinal Stromal Tumors|journal=The American Journal of Pathology|volume=156|issue=3|year=2000|pages=791–795|issn=00029440|doi=10.1016/S0002-9440(10)64946-2}}</ref> | ||
| Line 56: | Line 56: | ||
{{familytree/start |summary=Sample 1}} | {{familytree/start |summary=Sample 1}} | ||
{{familytree | | | | | | | | | | | | | A01 |A01=Gastrointestinal stromal tumors}} | {{familytree | | | | | | | | | | | | | | A01 |A01=Gastrointestinal stromal tumors}} | ||
{{familytree | | | | | | |,|-|-|-|-|-|-|+|-|-|-|-|-|-|.| }} | {{familytree | | | | | | | | | | | | | | |!| | | | | | | | }} | ||
{{familytree | | | | | | C01 | | | | | C02 | | | | | C03 |C01=KIT gene mutation|C02=PDGFRA mutation|C03=Wild type (absence of KIT/PDGFRA)}} | {{familytree | | | | | | | | | | | | | | A02 |A02=Uncontrolled cell proliferation can be from}} | ||
{{familytree | | |,|-|-|-|^|-|-|-|.| | | | | | | | | |!| |}} | {{familytree | | | | | | |,|-|-|-|-|-|-|-|+|-|-|-|-|-|-|.| }} | ||
{{familytree | | D01 | | | | | | D02 | | | | | | | | {{familytree | | | | | | C01 | | | | | | C02 | | | | | C03 |C01=KIT gene mutation|C02=PDGFRA mutation|C03=Wild type (absence of KIT/PDGFRA mutation)}} | ||
{{familytree | | |!| | | | | | | |!| | | | | | | | | |!| |}} | {{familytree | | |,|-|-|-|^|-|-|-|.| | | |!| | | | | | |!| |}} | ||
{{familytree | | E01 | | | | | | E02 | | | | | | | | {{familytree | | D01 | | | | | | D02 | | D03 | | | | | D04 |D01=Exon 9,13 & 17|D02=Exon 11|D03= Exon 18|D04=Mutant succinate dehydrogenase}} | ||
{{familytree | | | | | | | | | | | | | | | | | | | | |!| |}} | {{familytree | | |!| | | | | | | |!| | | |!| | | | | | |!| |}} | ||
{{familytree | | | | | | | | | | | | | | | | | | | | F01 |F01=Defective oxidative phosphorylation}} | {{familytree | | E01 | | | | | | E02 | | E03 | | | | | E04 |E01=Uncontrolled KIT signalling|E02=KIT receptor mutation & uncontrolled activation|E03=Uncontrolled activation|E04=Dysfunction of electron transport mitochondria}} | ||
{{familytree | | | | | | | | | | | | | | | | | | | | |!| |}} | {{familytree | | | | | | | | | | | | | | | | | | | | | |!| |}} | ||
{{familytree | | | | | | | | | | | | | | | | | | | | G01 |G01=Abnormal stabilization of HIF}} | {{familytree | | | | | | | | | | | | | | | | | | | | | F01 |F01=Defective oxidative phosphorylation}} | ||
{{familytree | | | | | | | | | | | | | | | | | | | | | |!| |}} | |||
{{familytree | | | | | | | | | | | | | | | | | | | | | G01 |G01=Abnormal stabilization of HIF transcription factor<br>(Hypoxia-inducible factor-1)}} | |||
{{familytree/end}} | {{familytree/end}} | ||
Latest revision as of 19:06, 22 February 2019
|
Gastrointestinal stromal tumor Microchapters |
|
Differentiating Gastrointestinal stromal tumor from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Gastrointestinal stromal tumor pathophysiology On the Web |
|
American Roentgen Ray Society Images of Gastrointestinal stromal tumor pathophysiology |
|
Directions to Hospitals Treating Gastrointestinal stromal tumor |
|
Risk calculators and risk factors for Gastrointestinal stromal tumor pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Akshun Kalia M.B.B.S.[2]
Overview
Gastrointestinal stromal tumors (GISTs) are rare but the most common mesenchymal (nonepithelial) tumors of the gastrointestinal tract. GISTs are derived from the interstitial cells of Cajal or undifferen2tiated precursor cells that finally develop into interstitial cells of Cajal. GIST tumors can either be benign tumors or massive malignant tumors with widespread metastasis. They can occur in any part of the gastrointestinal tract with the most common location as stomach. GIST (tumors) can grow as an endophytic or exophytic lesions. Genes involved in the pathogenesis of gastrointestinal stromal tumors include mutations in c-Kit gene and PDGFRA (platelet derived growth factor receptor-alpha) gene. Both Kit gene and PDGFRA are tyrosine kinase receptors and control cell proliferation. Mutation in c-Kit gene and PDGFRA leads to inhibition of apoptosis and uncontrolled cell proliferation. In some rare cases where the patient do not exhibit the typical mutation in c-Kit and PDGFRA, mutations in succinate dehydrogenase (SDH) have been reported. Conditions associated with GIST include urticaria pigmentosa, neurofibromatosis type 1, and Carney-Stratakis syndrome. On gross pathology, GISTs have a rounded appearance with areas of hemorrhage. On microscopic histopathological analysis, GISTs are cellular tumors arising from muscularis propria and composed of spindle cells (70%), epithelioid cells (20%) or either one of them.
Pathophysiology
- Gastrointestinal stromal tumors (GISTs) are rare but the most common mesenchymal (nonepithelial) tumors of the gastrointestinal tract.[1]
- GIST tumors can either be benign tumors or massive malignant tumors with widespread metastasis.
- Earlier GIST were thought to arise from the submucosal or smooth muscle cells of the GI tract. However recent research have proved that GISTs are derived from the interstitial cells of Cajal or undifferentiated precursor cells that finally develop into interstitial cells of Cajal.[2][3]
- Interstitial cells of Cajal (ICC) are a normal part of myenteric plexus and the autonomic nervous system of the intestine.
- Interstitial cells of Cajal serve as a pacemaker of intestine and controls intestinal motility.
- Molecular analysis has shown that GISTs arising from the interstitial cells of Cajal, stain positive for CD117 (c-KIT) in 90% cases and CD34 in 70% of cases. Around 5% of the cases are positive for PDGFRA. The rest of the cases are defined as wild type (negative for both CD117 and PDGFRA).
- CD117 is encoded by the KIT gene. Other names for CD117 include proto-oncogene c-Kit and tyrosine kinase receptor Kit.
- CD34 is the myeloid progenitor cell antigen and also known as hematopoietic progenitor cell antigen CD34.
- PDGFRA is platelet derived growth factor receptor-alpha and is a tyrosine kinase receptor.
- GIST can occur in any part of the gastrointestinal tract. Thus, GIST vary considerably in their presentation and clinical course, ranging from being asymptomatic to presenting with severe signs and symptoms of bleeding, abdominal pain and perforation.[4]
- The most common location for GIST is the stomach with the second most common location as the small intestine.
- Less frequent sites of occurrence include the colon, rectum and esophagus.
- Rare sites include pancreas, peritoneum, omentum, or mesentery.
- GIST (tumors) can grow as an endophytic or exophytic lesions.
- GIST have a variable malignant potential.[5]
- About 40% of GISTs that are localized at initial diagnosis give rise to metastasis.
- Of all GIST, 10%-20% present with distant metastasis with the liver being the most frequent site of metastasis.[6][7]
- Other common sites of metastasis include the bone, peritoneum, retroperitoneum, lung, and pleura.
Genetics
Genes involved in the pathogenesis of gastrointestinal stromal tumors include mutations in c-Kit gene and PDGFRA (platelet derived growth factor receptor-alpha) gene. In some rare cases where the patient do not exhibit the typical c-Kit and PDGFRA mutation, mutation in succinate dehydrogenase (SDH) have been reported. Rare genes involved include BRAF kinase, and protein kinase C. The majority of GISTs are sporadic in origin. [8][9][10][11]
- The c-kit gene is a proto-oncogene and located on chromosome 4q11-12 (long (q) arm of chromosome 4 at position 12).
- The c-kit gene encodes for KIT protein which is a transmembrane tyrosine kinase.
- The KIT protein is located on the cell membrane of certain cell types.
- Stem cell factor is the ligand that binds to KIT protein, which in turn leads to activation of KIT protein.
- Upon activation, the KIT protein leads to activation of other intracellular proteins by a process known as phosphorylation (which involves adding oxygen and phosphorus at specific positions).
- The activation of these intracellular proteins such as (MAP kinase and RAS) plays a vital role in multiple signaling pathways.
- The signaling pathways stimulated by the KIT protein control many important cellular processes such as cell growth and proliferation.
- In addition, KIT protein signaling also has a role in the development of gastrointestinal tract cells known as interstitial cells of Cajal.
- The most commonly observed mutation site in c-Kit gene involves exon 11 leading to a gain-of-function mutation. Less common sites include exons 9 and 13.
- Gain of function mutation leads to overexpression and autophosphorylation of c-Kit that leads to inhibition of apoptosis and uncontrolled cell proliferation.
- Almost 90-95% of patients with GIST have mutated c-Kit gene.
- C-Kit (a tyrosine kinase growth factor receptor) is also the target of medical therapy in GIST; ST-571 (Imatinib; Glivec).
- About 10% cases of GIST are associated with PDGFRA gene.
- The PDGFRA gene is located on chromosome 4q11-12 (long (q) arm of chromosome 4 at position 12).
- The PDGFRA gene encodes for the protein; platelet-derived growth factor receptor alpha (PDGFRA), which belongs to a family of proteins known as receptor tyrosine kinases.
- The platelet-derived growth factor is the ligand that binds to PDGFRA ,which in turn activates the PDGFRA.
- Upon activation, the PDGFRA leads to activation of other intracellular proteins by a process known as phosphorylation (same as c-Kit explained above).
- The activation of these intracellular proteins such as (MAP kinase and RAS) plays a vital role in multiple signaling pathways.
- The multiple signaling pathways stimulated by PDGFRA gene control many important cellular processes such as cell growth and proliferation.
- The most commonly observed mutation site in PDGFRA gene involves exon 18.
- As a result of mutation, the PDGFRA gene gets activated on its own and leads to inhibition of apoptosis and uncontrolled cell proliferation.
| Gastrointestinal stromal tumors | |||||||||||||||||||||||||||||||||||||||||||||||
| Uncontrolled cell proliferation can be from | |||||||||||||||||||||||||||||||||||||||||||||||
| KIT gene mutation | PDGFRA mutation | Wild type (absence of KIT/PDGFRA mutation) | |||||||||||||||||||||||||||||||||||||||||||||
| Exon 9,13 & 17 | Exon 11 | Exon 18 | Mutant succinate dehydrogenase | ||||||||||||||||||||||||||||||||||||||||||||
| Uncontrolled KIT signalling | KIT receptor mutation & uncontrolled activation | Uncontrolled activation | Dysfunction of electron transport mitochondria | ||||||||||||||||||||||||||||||||||||||||||||
| Defective oxidative phosphorylation | |||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal stabilization of HIF transcription factor (Hypoxia-inducible factor-1) | |||||||||||||||||||||||||||||||||||||||||||||||
Associated Conditions
- Urticaria pigmentosa
- Neurofibromatosis type 1
- Carney-Stratakis syndrome
Gross Pathology
On gross pathology, GISTs have the following findings:
- Rounded appearance with areas of hemorrhage.
- Large tumors may have necrosis or cystic change.
- Variable size (ranging from 1 to 30cms).

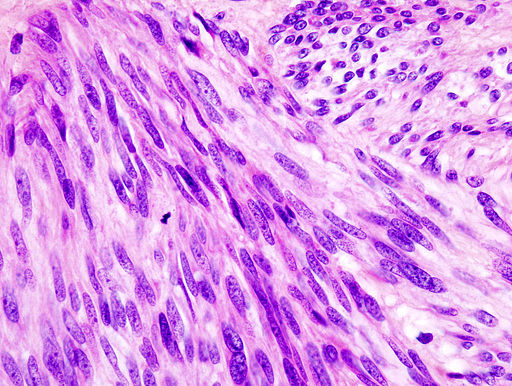
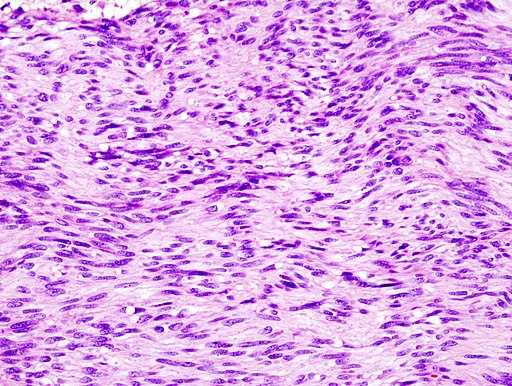
Microscopic Pathology
On microscopic histopathological analysis, GISTs are cellular tumors arising from muscularis propria and composed of:[12]
- Spindle cells (60%-80%): Spindle cells have a fascicular or whorled like appearance and are made of multiple compact cells with minimal stroma and eosinophilic, basophilic or amphophilic cytoplasm. The CD117 expression in spindle cells are generally diffuse and strong.
- Epithelioid cells (20%-30%): Epithelioid tumors are clearly defined with an abundant cytoplasm which is amphophilic to clear. The CD117 expression in epithelioid cells is generally focal and weakly positive.
- Spindle cells or epithelioid cells (10%).
References
- ↑ Miettinen M, Lasota J (2001). "Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis". Virchows Arch. 438 (1): 1–12. PMID 11213830.
- ↑ Miettinen M, Lasota J (2006). "Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis". Arch Pathol Lab Med. 130 (10): 1466–78. PMID 17090188.
- ↑ Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM (1998). "Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal". Am. J. Pathol. 152 (5): 1259–69. PMC 1858579. PMID 9588894.
- ↑ Reith JD, Goldblum JR, Lyles RH, Weiss SW (2000). "Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome". Mod. Pathol. 13 (5): 577–85. doi:10.1038/modpathol.3880099. PMID 10824931.
- ↑ Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, Bordoni A, Magnusson MK, Linke Z, Sufliarsky J, Federico M, Jonasson JG, Dei Tos AP, Rutkowski P (2012). "Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts". Lancet Oncol. 13 (3): 265–74. doi:10.1016/S1470-2045(11)70299-6. PMID 22153892.
- ↑ Woodall CE, Brock GN, Fan J, Byam JA, Scoggins CR, McMasters KM, Martin RC (2009). "An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system". Arch Surg. 144 (7): 670–8. doi:10.1001/archsurg.2009.108. PMID 19620548.
- ↑ Emile JF, Brahimi S, Coindre JM, Bringuier PP, Monges G, Samb P, Doucet L, Hostein I, Landi B, Buisine MP, Neuville A, Bouché O, Cervera P, Pretet JL, Tisserand J, Gauthier A, Le Cesne A, Sabourin JC, Scoazec JY, Bonvalot S, Corless CL, Heinrich MC, Blay JY, Aegerter P (2012). "Frequencies of KIT and PDGFRA mutations in the MolecGIST prospective population-based study differ from those of advanced GISTs". Med. Oncol. 29 (3): 1765–72. doi:10.1007/s12032-011-0074-y. PMID 21953054.
- ↑ Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA (2003). "Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor". J. Clin. Oncol. 21 (23): 4342–9. doi:10.1200/JCO.2003.04.190. PMID 14645423.
- ↑ Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y (1998). "Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors". Science. 279 (5350): 577–80. PMID 9438854.
- ↑ Duensing, Anette; Medeiros, Fabiola; McConarty, Bryna; Joseph, Nora E; Panigrahy, Dipak; Singer, Samuel; Fletcher, Christopher DM; Demetri, George D; Fletcher, Jonathan A (2004). "Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs)". Oncogene. 23 (22): 3999–4006. doi:10.1038/sj.onc.1207525. ISSN 0950-9232.
- ↑ Lux, Marcia L.; Rubin, Brian P.; Biase, Tara L.; Chen, Chang-Jie; Maclure, Timothy; Demetri, George; Xiao, Sheng; Singer, Samuel; Fletcher, Christopher D.M.; Fletcher, Jonathan A. (2000). "KIT Extracellular and Kinase Domain Mutations in Gastrointestinal Stromal Tumors". The American Journal of Pathology. 156 (3): 791–795. doi:10.1016/S0002-9440(10)64946-2. ISSN 0002-9440.
- ↑ "Gastrointestinal stromal tumour".
- ↑ By No machine-readable author provided. KGH assumed (based on copyright claims). [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons
- ↑ By No machine-readable author provided. KGH assumed (based on copyright claims). [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons