Small cell carcinoma of the lung diagnostic study of choice: Difference between revisions
No edit summary |
|||
| (16 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Small cell carcinoma of the lung}} | {{Small cell carcinoma of the lung}} | ||
{{CMG}}; {{AE}} | {{CMG}}; {{AE}} {{SH}}{{Rim}} | ||
== Overview == | == Overview == | ||
The confirmation of the diagnosis of SCLC relies on the histopathological findings of the tumor [[biopsy]]. All patients with confirmed diagnosis of SCLC by [[histopathological]] findings should undergo a [[CT scan]] of the [[abdomen]] for staging purposes. [[Computed tomography|CT scan]] of the [[abdomen]] helps identify [[metastasis]] to organs, such as the [[liver]] or the [[adrenal glands]]. Staging schemes for small cell lung cancer (SCLC) have been developed by the Veterans Administration Lung Study Group (VALG), the American Joint Committee on Cancer (AJCC), and the National Comprehensive Cancer Network (NCCN). | |||
== Diagnostic Study of Choice == | == Diagnostic Study of Choice == | ||
===Biopsy=== | |||
* The confirmation of the diagnosis of SCLC relies on the histopathological findings of the tumor [[biopsy]].<ref name="NCCN">[http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 2.2014] </ref> | |||
* In SCLC, the [[Tumor cell|tumor cells]] are small and round, but they can sometimes be ovoid or spindle shaped. They have a scant [[cytoplasm]] with a high [[mitotic]] count and a hyperchromatic [[nuclei]]. Nearly all SCLC are immunoreactive for [[keratin]], [[thyroid transcription factor 1]], and [[Epithelial cells|epithelial]] membrane [[antigen]]. [[Neuroendocrine]] and [[neural]] [[differentiation]] result in the expression of molecules like [[Dopamine beta-hydroxylase|dopa-decarboxylase]], [[calcitonin]], [[neuron-specific enolase]], [[chromogranin A]], [[CD56]] (also known as nucleosomal [[histone]] kinase 1 or [[neural]]-[[cell]] adhesion molecule), [[gastrin]]-releasing [[peptide]], and [[insulin-like growth factor 1]]. One or more markers of [[neuroendocrine]] differentiation can be found in approximately 75% of SCLC.<ref name="NCI">National Cancer Institute: PDQ® Small Cell Lung Cancer Treatment. Bethesda, MD: National Cancer Institute. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional.</ref> | |||
=== | {| class="wikitable" | ||
|[[Image:Lung small cell carcinoma (1) by core needle biopsy.jpg|300px|thumb| Histopathologic image of small cell carcinoma of the lung. CT-guided core needle biopsy. H & E stain.By No machine-readable author provided. KGH assumed (based on copyright claims),via Wikimedia Commons <ref>href="http://www.gnu.org/copyleft/fdl.html">GFDL<nowiki></a></nowiki> or <nowiki><a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a></nowiki>], <nowiki><a href="https://commons.wikimedia.org/wiki/File%3ALung_small_cell_carcinoma_(1)_by_core_needle_biopsy.jpg"></nowiki></ref>]] | |||
* | |[[Image:Lung small cell cancer 01.jpeg|300px|thumb| Micrograph of a small-cell carcinoma of the lung showing cells with nuclear moulding, minimal amount of cytoplasm and stippled chromatin. FNA specimen. Field stain.By No machine-readable author provided. KGH assumed (based on copyright claims), via Wikimedia Commons <ref>href="http://www.gnu.org/copyleft/fdl.html">GFDL ="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0], href="https://commons.wikimedia.org/wiki/File%3ALung_small_cell_carcinoma_(1)_by_core_needle_biopsy.jpg"></ref>]] | ||
* | |- [[Image:Lung small cell cancer 03.jpeg|300px|thumb| Anaplastic (microcellular, oat cell) carcinoma from the lung., via Wikimedia Commons <ref>href="https://commons.wikimedia.org/wiki/File:Carcinoma_microcellulare_oatcell_carcinoma_or_anaplastic_carcinoma_(lung)H%26E_magn_200x.jpg</ref>]] | ||
* | | | ||
| | |||
* | |} | ||
* | |||
* [ | ===CT=== | ||
* The | Chest [[Computed tomography|CT scan]], preferably with [[intravenous]] [[contrast]] administration, may be helpful in the [[diagnosis]] of small cell carcinoma. Findings on [[Computed tomography|CT scan]] suggestive of small cell carcinoma include:<ref name="NCCN">[http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 2.2014] </ref> | ||
* | *[[Hilum|Hilar]] mass | ||
* | *[[Mediastinum|Mediastinal]] involvement | ||
* [ | *Numerous [[lymphadenopathy]] | ||
*Direct infiltration of adjacent structures | |||
*[[Necrosis]] | |||
*[[Hemorrhage]] | |||
* | *The most common cause of [[SVC obstruction]] is SCLC, because of both compression or [[thrombosis]] and or direct infiltration. | ||
*[[CT-scans|CT]] is used to stage small cell lung cancer. | |||
*CT scan of the abdomen helps identify [[metastasis]] to organs, such as the [[liver]] or the [[adrenal glands]]. | |||
*[[Brain]] imaging is also mandatory for staging however a brain [[MRI]] is preferred over brain [[CT scan]] due to its superior [[sensitivity]] for the detection of [[brain]] [[metastasis]]. | |||
*[[PET]] [[CT-scans|CT]] scan should be performed if limited stage small cell lung cancer is suspected. | |||
===Staging=== | |||
The Veterans Administration Lung Study Group (VALG) staging, also known as VA staging, is an old staging system that has been previously used in most clinical trials. Shown below is a table depicting the VA staging system which classifies SCLC into two stages.<ref name="pmid12234695">{{cite journal| author=Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG et al.| title=Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? | journal=Lung Cancer | year= 2002 | volume= 37 | issue= 3 | pages= 271-6 | pmid=12234695 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12234695 }} </ref> | |||
{| style="cellpadding=0; cellspacing= 0; width: 600px;" | |||
{| | |||
|- | |- | ||
| style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''Stage'''||style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''Characteristics''' | |||
| style=" | |||
|- | |- | ||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=left |'''Limited SCLC''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=left | | |||
| style=" | * Limited SCLC is characterized by the strict involvement of the ipsilateral lung. | ||
| style="background: #DCDCDC; padding: 5px; | |- | ||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=left |'''Extensive SCLC''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=left | | |||
* Extensive SCLC extends beyond the ipsilateral [[lung]] and may involve the contralateral lung, or can be associated with [[pleural effusion]], [[pericardial effusion]], or hematogenous spread. | |||
|} | |} | ||
===== | ===AJCC and TNM Staging=== | ||
The | Shown below is a table summarizing the staging of lung cancer according to the American Joint Committee on Cancer (AJCC). This staging scheme is the same for both SCLC and [[non small cell lung cancer]].<ref name="pmid18090577">{{cite journal| author=Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K et al.| title=The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. | journal=J Thorac Oncol | year= 2007 | volume= 2 | issue= 12 | pages= 1067-77 | pmid=18090577 | doi=10.1097/JTO.0b013e31815bdc0d | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18090577 }} </ref> | ||
''For more information about the TNM staging, click '''[[lung cancer staging|here]]'''.'' | |||
=== | {| style="cellpadding=0; cellspacing= 0; width: 600px;" | ||
|- | |||
| style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''Stage'''||style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''T'''||style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''N'''||style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''M''' | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center |'''Occult carcinoma''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center|TX|| style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N0|| style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center |'''Stage 0''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |Tis ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center | N0 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center |'''Stage IA''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T1 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N0 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center |'''Stage IB''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T2 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N0 ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center | M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center |'''Stage IIA''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T1 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N1 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center rowspan="2"|'''Stage IIB''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T2 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N1 ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center | M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T3 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N0 || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center rowspan="2" |'''Stage IIIA''' ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T1, T2|| style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N2 ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T3|| style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N1, N2 ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center rowspan="2"|'''Stage IIIB''' ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |Any T|| style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |N3 ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |T4 ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center|Any N ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M0 | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=center |'''Stage IV'''|| style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |Any T ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |Any N ||style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=center |M1 | |||
|} | |||
===NCCN Staging=== | |||
The National Comprehensive Cancer Network (NCCN) staging system combines the staging scheme of the AJCC and that of the VALG. Although the AJCC staging scheme is newer than that of the VALG, clinicians commonly use the VALG staging system because it has been commonly referred to in clinical trials. Shown below is a table depicting the NCNN staging which classifies SCLC into two stages.<ref name="NCCN">[http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 2.2014] </ref> | |||
* [ | {| style="cellpadding=0; cellspacing= 0; width: 600px;" | ||
** | |- | ||
* | | style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''Stage'''||style="padding: 0 5px; font-size: 100%; background: #4682B4; color: #FFFFFF" align=center |'''Characteristics''' | ||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=left |'''Limited SCLC''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=left| | |||
* Limited SCLC includes lung cancer cases of stage I, II, or III which can be treated with [[radiation therapy]]. | |||
* This stage does not include tumor [[Lung cancer staging#T:Primary Tumor|T3]] or [[Lung cancer staging#T:Primary Tumor|T4]] with multiple lung nodules, as well as tumor or [[lymph node]]s that are too large to fit in the radiation plan. | |||
|- | |||
|style="font-size: 100; padding: 0 5px; background: #DCDCDC" align=left |'''Extensive SCLC''' || style="font-size: 100; padding: 0 5px; background: #F5F5F5" align=left | | |||
* Extensive SCLC includes all SCLC categorized as stage IV, '''or''' | |||
* Tumor [[Lung cancer staging#T:Primary Tumor|T3]] or [[Lung cancer staging#T:Primary Tumor|T4]] with multiple lung nodules, '''or''' | |||
* [[Tumor]] or [[lymph node]]s that are too large to fit in the radiation plan | |||
|} | |||
==References== | |||
{{Reflist|2}} | |||
[[Category:Disease]] | |||
[[Category:Types of cancer]] | |||
[[Category:Pulmonology]] | |||
[[Category:Lung cancer]] | |||
{{WikiDoc Help Menu}} | |||
{{ | {{WikiDoc Sources}} | ||
{{ | [[Category:Up-To-Date]] | ||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
[[Category:Pulmonology]] | |||
[[Category:Surgery]] | |||
Latest revision as of 22:32, 3 September 2019
|
Small Cell Carcinoma of the Lung Microchapters |
|
Differentiating Small Cell Carcinoma of the Lung from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Small cell carcinoma of the lung diagnostic study of choice On the Web |
|
American Roentgen Ray Society Images of Small cell carcinoma of the lung diagnostic study of choice |
|
FDA on Small cell carcinoma of the lung diagnostic study of choice |
|
CDC on Small cell carcinoma of the lung diagnostic study of choice |
|
Small cell carcinoma of the lung diagnostic study of choice in the news |
|
Blogs on Small cell carcinoma of the lung diagnostic study of choice |
|
Directions to Hospitals Treating Small cell carcinoma of the lung |
|
Risk calculators and risk factors for Small cell carcinoma of the lung diagnostic study of choice |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Dildar Hussain, MBBS [2]Rim Halaby, M.D. [3]
Overview
The confirmation of the diagnosis of SCLC relies on the histopathological findings of the tumor biopsy. All patients with confirmed diagnosis of SCLC by histopathological findings should undergo a CT scan of the abdomen for staging purposes. CT scan of the abdomen helps identify metastasis to organs, such as the liver or the adrenal glands. Staging schemes for small cell lung cancer (SCLC) have been developed by the Veterans Administration Lung Study Group (VALG), the American Joint Committee on Cancer (AJCC), and the National Comprehensive Cancer Network (NCCN).
Diagnostic Study of Choice
Biopsy
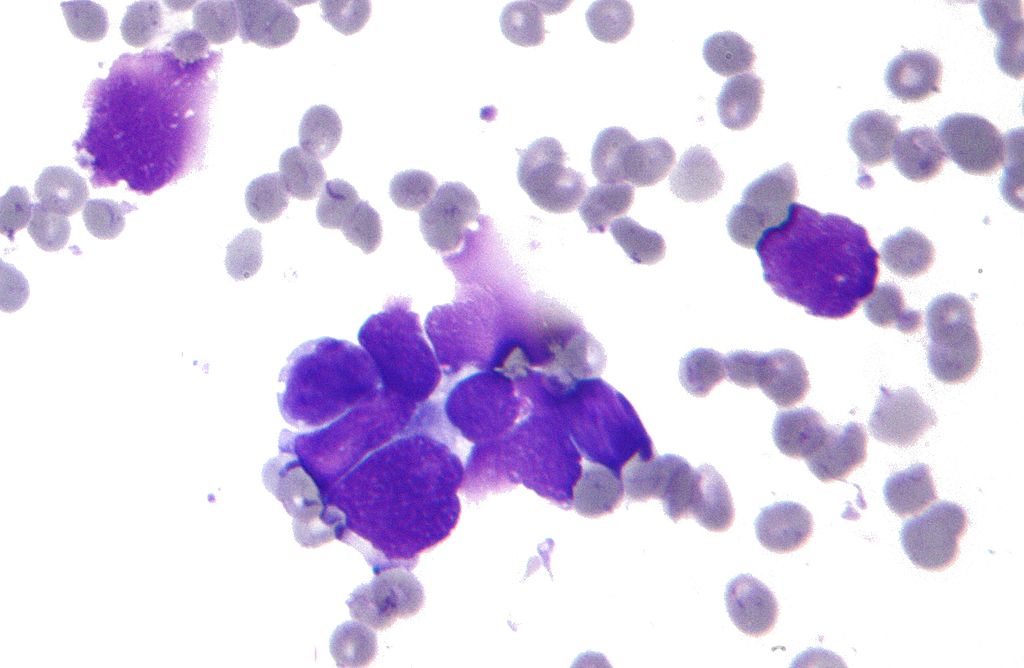
- The confirmation of the diagnosis of SCLC relies on the histopathological findings of the tumor biopsy.[1]
- In SCLC, the tumor cells are small and round, but they can sometimes be ovoid or spindle shaped. They have a scant cytoplasm with a high mitotic count and a hyperchromatic nuclei. Nearly all SCLC are immunoreactive for keratin, thyroid transcription factor 1, and epithelial membrane antigen. Neuroendocrine and neural differentiation result in the expression of molecules like dopa-decarboxylase, calcitonin, neuron-specific enolase, chromogranin A, CD56 (also known as nucleosomal histone kinase 1 or neural-cell adhesion molecule), gastrin-releasing peptide, and insulin-like growth factor 1. One or more markers of neuroendocrine differentiation can be found in approximately 75% of SCLC.[2]
 |
 |
CT
Chest CT scan, preferably with intravenous contrast administration, may be helpful in the diagnosis of small cell carcinoma. Findings on CT scan suggestive of small cell carcinoma include:[1]
- Hilar mass
- Mediastinal involvement
- Numerous lymphadenopathy
- Direct infiltration of adjacent structures
- Necrosis
- Hemorrhage
- The most common cause of SVC obstruction is SCLC, because of both compression or thrombosis and or direct infiltration.
- CT is used to stage small cell lung cancer.
- CT scan of the abdomen helps identify metastasis to organs, such as the liver or the adrenal glands.
- Brain imaging is also mandatory for staging however a brain MRI is preferred over brain CT scan due to its superior sensitivity for the detection of brain metastasis.
- PET CT scan should be performed if limited stage small cell lung cancer is suspected.
Staging
The Veterans Administration Lung Study Group (VALG) staging, also known as VA staging, is an old staging system that has been previously used in most clinical trials. Shown below is a table depicting the VA staging system which classifies SCLC into two stages.[6]
| Stage | Characteristics |
| Limited SCLC |
|
| Extensive SCLC |
|
AJCC and TNM Staging
Shown below is a table summarizing the staging of lung cancer according to the American Joint Committee on Cancer (AJCC). This staging scheme is the same for both SCLC and non small cell lung cancer.[7]
For more information about the TNM staging, click here.
| Stage | T | N | M |
| Occult carcinoma | TX | N0 | M0 |
| Stage 0 | Tis | N0 | M0 |
| Stage IA | T1 | N0 | M0 |
| Stage IB | T2 | N0 | M0 |
| Stage IIA | T1 | N1 | M0 |
| Stage IIB | T2 | N1 | M0 |
| T3 | N0 | M0 | |
| Stage IIIA | T1, T2 | N2 | M0 |
| T3 | N1, N2 | M0 | |
| Stage IIIB | Any T | N3 | M0 |
| T4 | Any N | M0 | |
| Stage IV | Any T | Any N | M1 |
NCCN Staging
The National Comprehensive Cancer Network (NCCN) staging system combines the staging scheme of the AJCC and that of the VALG. Although the AJCC staging scheme is newer than that of the VALG, clinicians commonly use the VALG staging system because it has been commonly referred to in clinical trials. Shown below is a table depicting the NCNN staging which classifies SCLC into two stages.[1]
| Stage | Characteristics |
| Limited SCLC |
|
| Extensive SCLC |
|
References
- ↑ 1.0 1.1 1.2 NCCN Clinical Practice Guidelines in Oncology. Small Cell Lung Cancer, version 2.2014
- ↑ National Cancer Institute: PDQ® Small Cell Lung Cancer Treatment. Bethesda, MD: National Cancer Institute. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/small-cell-lung/healthprofessional.
- ↑ href="http://www.gnu.org/copyleft/fdl.html">GFDL</a> or <a href="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0</a>], <a href="https://commons.wikimedia.org/wiki/File%3ALung_small_cell_carcinoma_(1)_by_core_needle_biopsy.jpg">
- ↑ href="http://www.gnu.org/copyleft/fdl.html">GFDL ="http://creativecommons.org/licenses/by-sa/3.0/">CC-BY-SA-3.0], href="https://commons.wikimedia.org/wiki/File%3ALung_small_cell_carcinoma_(1)_by_core_needle_biopsy.jpg">
- ↑ href="https://commons.wikimedia.org/wiki/File:Carcinoma_microcellulare_oatcell_carcinoma_or_anaplastic_carcinoma_(lung)H%26E_magn_200x.jpg
- ↑ Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG; et al. (2002). "Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease?". Lung Cancer. 37 (3): 271–6. PMID 12234695.
- ↑ Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K; et al. (2007). "The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer". J Thorac Oncol. 2 (12): 1067–77. doi:10.1097/JTO.0b013e31815bdc0d. PMID 18090577.