|
|
| (10 intermediate revisions by one other user not shown) |
| Line 1: |
Line 1: |
| <div style="-webkit-user-select: none;">
| | __NOTOC__ |
| {|class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right; | | {{Secondary hyperaldosteronism}} |
| | == Overview == |
| | Secondary hyperaldosteronism is a disease of increasing aldosterone or other mineralocorticoid levels. The resulting Na+ retention produces [[hypertension]], and elevated K+ excretion may cause [[hypokalemia]]. |
| | |
| | == Pathophysiology == |
| | |
| | === Renin-angiotensin system components === |
| | [[Renin]]: |
| | * In each [[kidney]] there is a specialized system called [[juxtaglomerular apparatus]], which is located in [[afferent arteriole]] of each [[glomerulus]]. |
| | * [[Juxtaglomerular apparatus]] synthesizes prorenin, and then later it converts into [[renin]] with mediation of a [[proteolytic enzyme]]. |
| | * [[Renin]] stores in and then may be released from secretory [[granules]], in response to various factors. |
| | * [[Renin]] releasing starts a cascade of steps, and the first step is the cleavage of the [[angiotensin]] I from [[angiotensinogen]] ([[renin]] substrate). |
| | * [[Angiotensinogen]] is an alpha-2-globulin that is produced in the [[liver]] and [[kidney]] and other organs. |
| | * The first step is the rate-limiting step of the [[Renin-angiotensin system|renin-angiotensin]] cascade. |
| | * Most important stimuli to [[renin]] secretion are : |
| | ** [[Renal]] hypoperfusion, due to hypotension or [[volume depletion]] |
| | ** Increased [[sympathetic]] activity. |
| | |
| | {| class="wikitable" |
| | !Renin-angiotensin system components |
| | !Secretion control |
| | !Measurement |
| | !Inhibition |
| | |- |
| | |Renin |
| | | |
| | * Baroreceptors (or stretch receptors) in the wall of the afferent arteriole |
| | |
| | * Cardiac and arterial baroreceptors, which regulate sympathetic neural activity and the level of circulating catecholamines, both of which enhance renin secretion via the beta-1-adrenergic receptors |
| | * The cells of the macula densa in the early distal tubule, which appear to be stimulated by a reduction in chloride delivery, particularly in the chloride concentration in the fluid delivered to this site |
| | | |
| | * The plasma renin activity (PRA) is an indirect measure of renin that employs an enzyme-kinetic bioassay, which measures its capacity to generate angiotensin I. The rate of angiotensin I production, and therefore PRA, is critically dependent upon the concentration of substrate in plasma (ie, angiotensinogen). |
| | |
| | * The plasma renin concentration (PRC) employs a direct immunosorbent assay that detects both prorenin and renin |
| | | |
| |- | | |- |
| | {{#ev:youtube|https://https://www.youtube.com/watch?v=JBfkGNr01V8|350}} | | |Angiotensinogen |
| | | |
| | | |
| | | |
| |- | | |- |
| | |Angiotensin-converting enzyme |
| | | |
| | | |
| | | |
| | |- |
| | |Angiotensin II |
| | | |
| | | |
| | | |
| | |- |
| | |Aldosterone |
| | | |
| | | |
| | | |
| |} | | |} |
|
| |
|
| __NOTOC__
| | ==== Basic physiology of aldosterone ==== |
| {{Secondary hyperaldosteronism}}
| |
| == Overview == | |
| Secondary hyperaldoseronism can caused by renin-produsing tumors or renal artery stenosis several . The resulting Na+ retention produces [[hypertension]], and elevated K+ excretion may cause [[hypokalemia]]. Patients with Conn's syndrome due to primary hyperaldosertonism may have an aldosterone producing adrenocortical adenoma (APA)- Classically referred to as Conn's syndrome, <sup>[[Primary hyperaldosteronism pathophysiology#cite note-pmid17492946-2|[2]]]</sup>, a unilateral hyperplasia, idiopathic hyperaldosteronism (IHA, also known as bilateral adrenal hyperplasia).<sup>[[Primary hyperaldosteronism pathophysiology#cite note-pmid26252618-3|[3]]],</sup>, familial forms (familial hyperaldosteronism types I, II, and III) have also been described, [[ectopic]] secretion of aldosterone (The [[Ovary|ovaries]] and [[kidneys]] are the 2 organs described in the literature that, in the setting of [[neoplastic disease]], can be [[ectopic]] sources of [[aldosterone]], but this is a rare occurrence).
| |
| == Pathophysiology ==
| |
| === Basic physiology of aldosterone ===
| |
| Circulating [[aldosterone]] is principally made in the [[zona glomerulosa]] of the [[adrenal cortex]] (outer layer of the cortex) by a cascade of [[enzyme]] steps leading to the conversion of [[cholesterol]] to [[aldosterone]]. | | Circulating [[aldosterone]] is principally made in the [[zona glomerulosa]] of the [[adrenal cortex]] (outer layer of the cortex) by a cascade of [[enzyme]] steps leading to the conversion of [[cholesterol]] to [[aldosterone]]. |
| * [[Aldosterone]]'s production is regulated at two critical [[enzyme]] steps: | | * [[Aldosterone]]'s production is regulated at two critical [[enzyme]] steps: |
| Line 21: |
Line 67: |
|
| |
|
|
| |
|
| [[Image:Renin-angiotensin system in man shadow.png|600px|center|Renin angiotensin system, by Mikael Häggström - https://commons.wikimedia.org/w/index.php?curid=8458370]] | | [[Image:Renin-angiotensin system in man shadow.png|600px|center|thumb|Renin angiotensin system, by Mikael Häggström - https://commons.wikimedia.org/w/index.php?curid=8458370]] |
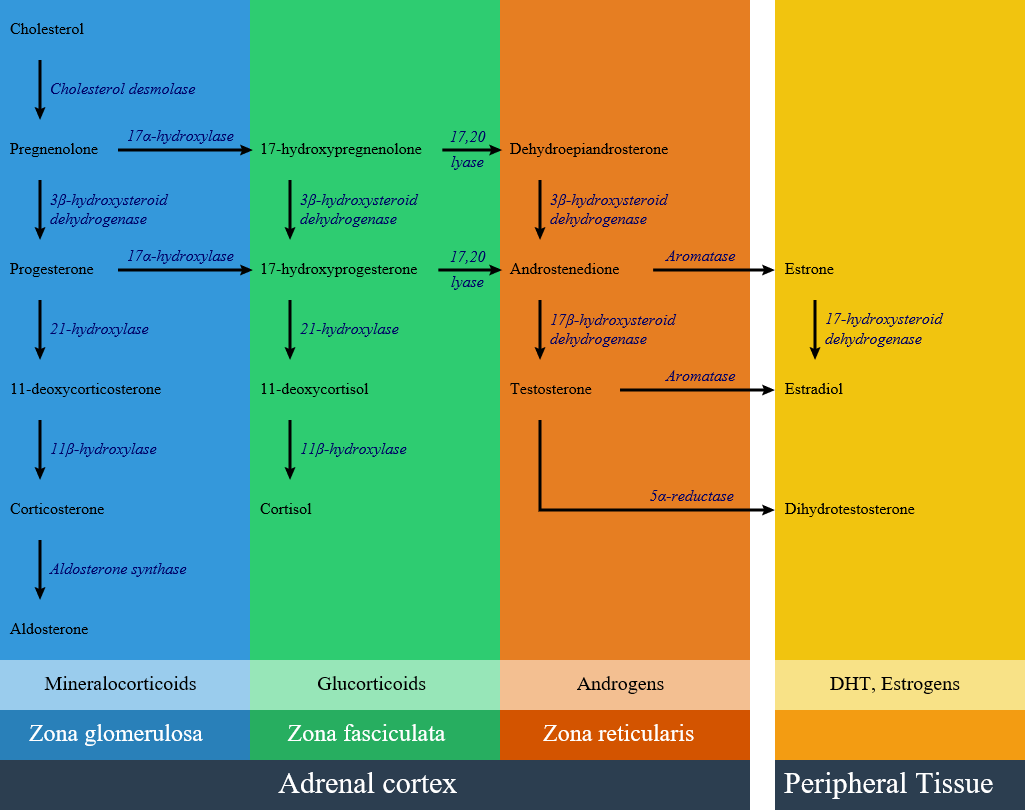
| [[Image:Adrenal Steroids.png|700px|center|Adrenal steroidogenesis]]
| |
|
| |
|
| ==Pathogenesis ==
| |
| Primary hyperaldoseronism (PA) features overproduction of [[aldosterone]] despite suppressed plasma [[renin]] activity (PRA). The resulting Na+ retention produces [[hypertension]], and elevated K+ excretion may cause [[hypokalemia]].
| |
| * Patients with primary hyperaldosertonism may have:
| |
| **Aldosterone producing adrenocortical adenoma (APA)- Classically referred to as Conn's syndrome.<ref name="pmid17492946">{{cite journal |vauthors=Young WF |title=Primary aldosteronism: renaissance of a syndrome |journal=Clin. Endocrinol. (Oxf) |volume=66 |issue=5 |pages=607–18 |year=2007 |pmid=17492946 |doi=10.1111/j.1365-2265.2007.02775.x |url=}}</ref>
| |
| **Unilateral [[hyperplasia]]
| |
| **Idiopathic hyperaldosteronism (IHA, also known as [[bilateral adrenal hyperplasia]]).<ref name="pmid26252618">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref>
| |
| **Familial forms (familial hyperaldosteronism types I, II, and III) have also been described.
| |
| **[[Ectopic]] secretion of [[aldosterone]] (The [[ovaries]] and [[kidneys]] are the 2 organs described in the literature that, in the setting of [[neoplastic disease]], can be [[ectopic]] sources of [[aldosterone]], but this is a rare occurrence.)
| |
| ==Genetics ==
| |
| '''<u>1. Aldosterone Producing Adenoma (APA)</u>'''
| |
|
| |
|
| APAs are typically [[solitary]], well circumscribed tumors which can cause [[aldosterone]] hypersecretion.
| | [[image:Adrenal Steroids.png|thumb|600px|center|frame|Adrenal steroid synthesis pathways in adrenal cortex and related enzymes <ref name="urlFile:Adrenal Steroids Pathways.svg - Wikimedia Commons">{{cite web |url=https://commons.wikimedia.org/wiki/File:Adrenal_Steroids_Pathways.svg|title=File:Adrenal Steroids Pathways.svg - Wikimedia Commons |format= |work= |accessdate=}}</ref>]] |
|
| |
|
| ===== Somatic mutations ===== | | ==Pathogenesis == |
| * Primary hyperaldosteronism producing aldosterone-producing adenomas (APAs) have [[mutations]] in [[Gene|genes]] encoding [[ion channels]]/pumps that change the [[intracellular]] [[calcium]] [[homeostasis]] and cause [[renin]]-independent [[aldosterone]] production through enhanced [[CYP11B2]] expression. Subcapsular aldosterone-producing cell clusters (APCCs) are [[CYP11B2]]-expressing clusters of cells that are found beneath the adrenal capsule but protrude into cortisol-producing [[Cell (biology)|cells]] that are negative for [[CYP11B2]] expression.
| | Secondary hyperaldosteronism syndrome is a disease of increasing aldosterone or other mineralocorticoid levels. The resulting Na+ retention produces [[hypertension]], and elevated K+ excretion may cause [[hypokalemia]]. |
| * APCCs are also frequently found in [[Adrenal gland|adrenal]] [[Tissue (biology)|tissue]] in close proximity to APA. | | Patients with Secondary hyperaldosertonism may have: |
| * The [[Renin-angiotensin system|renin-angiotensin axis]] is supressed in patients with APAs, pointing towards an autonomous, [[renin]]-independent production of [[aldosterone]] by APCCs. | | * [[Renin-producing tumors]] |
| * [[Somatic mutation|Somatic mutations]] in ''KCNJ5'', ''ATP1A1'', ''ATP2B3'', and ''CACNA1D'' are found in approximately 50 percent of APAs . | | * [[Renal artery stenosis]] |
| | * [[Cushing syndrome]] |
| | * [[Liddle's syndrome]] |
| | * Ectopic [[ACTH]] production |
| | * [[Licorice]] ingestion |
| | * Other mineralocorticoids excess: |
| | ** [[11β-hydroxylase deficiency|11 beta hydroxylase deficiency]] |
| | ** [[17 alpha-hydroxylase deficiency|17 alpha hydroxylase deficiency]] |
| | ** [[apparent mineralocorticoid excess]] |
|
| |
|
| =====Gain of function mutations(KCNJ5, CACNA1D, CTNNB1 mutations)===== | | ==Genetics == |
| * [[Inherited]] and [[Acquired disorder|acquired mutations]] in [[potassium]] inwardly rectifying channel, subfamily J, member 5 (''[[KCNJ5]])'' [[gene]], which codes for a K ion [[Ion channel|channel]] has been associated with autonomous [[cell proliferation]] in the [[adrenal cortex]]. <ref name="pmid21311022">{{cite journal |vauthors=Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP |title=K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension |journal=Science |volume=331 |issue=6018 |pages=768–72 |year=2011 |pmid=21311022 |pmc=3371087 |doi=10.1126/science.1198785 |url=}}</ref> Two somatic [[mutations]] in the K+ channel [[KCNJ5]] (G151R and L168R) cause ~40% of APA. <ref name="pmid262526182">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref> These [[mutations]] affect K ion selectivity leading to increased Na+ conductance and [[Depolarization|membrane depolarization]] resulting in activation of [[Voltage-gated ion channel|voltage-gated Ca2+channels]]. Increased [[intracellular]] Ca results in [[CYP11B2]] expression and release of [[aldosterone]] from the [[adrenal gland]]. Patients with [[KCNJ5]] [[mutations]] are more frequently female, diagnosed younger, and with higher minimal [[Blood plasma|plasma]] [[potassium]] concentrations. <ref name="pmid24866132">{{cite journal |vauthors=Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi T, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC |title=Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma |journal=Hypertension |volume=64 |issue=2 |pages=354–61 |year=2014 |pmid=24866132 |doi=10.1161/HYPERTENSIONAHA.114.03419 |url=}}</ref><ref name="pmid22628608">{{cite journal |vauthors=Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, Kurihara I, Williams TA, Giri JG, Bollag RJ, Edwards MA, Isales CM, Rainey WE |title=Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells |journal=J. Clin. Endocrinol. Metab. |volume=97 |issue=8 |pages=E1567–72 |year=2012 |pmid=22628608 |pmc=3410264 |doi=10.1210/jc.2011-3132 |url=}}</ref><ref name="pmid24037882">{{cite journal |vauthors=Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE |title=a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III |journal=J. Clin. Endocrinol. Metab. |volume=98 |issue=11 |pages=E1861–5 |year=2013 |pmid=24037882 |pmc=3816265 |doi=10.1210/jc.2013-2428 |url=}}</ref><ref name="pmid22315453">{{cite journal |vauthors=Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE |title=Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis |journal=Endocrinology |volume=153 |issue=4 |pages=1774–82 |year=2012 |pmid=22315453 |pmc=3320257 |doi=10.1210/en.2011-1733 |url=}}</ref>
| |
| * A [[germline mutation]] in the [[KCNJ5]] gene produces familial hyperaldosteronism type III.
| |
| *Gain-of-function [[mutation]] in the ''CACNA1D [[gene]].'' CACNA1D [[mutation]] leads to increased [[calcium]] influx through the mutant [[Ion channel|channel]] by shifting the voltage dependence of activation to less [[Depolarization|depolarized]] potentials and, in some cases, impairing inactivation.
| |
| *Activating somatic CTNNB1 [[mutations]], which mediate their effects through WnT signalling pathway have also been known to cause APA.<ref name="pmid26815163">{{cite journal |vauthors=Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P |title=Activating mutations in CTNNB1 in aldosterone producing adenomas |journal=Sci Rep |volume=6 |issue= |pages=19546 |year=2016 |pmid=26815163 |pmc=4728393 |doi=10.1038/srep19546 |url=}}</ref>
| |
| *CTNNB1 [[Mutation|mutations]] cause [[Adrenal cortex|adrenocortical cells]] to de-differentiate into their the precursor adrenal gonadal cell.
| |
| | |
| ==== Loss of function mutations(ATP1A1 and ATP2A3) ====
| |
| * Other genes implicated in development of APAs are loss-of-function [[mutations]] in [[ATP1A1]] and [[ATP2A3]] [[Gene|genes]].
| |
| *[[ATP1A1]] [[Mutation|mutations]] lead to permeability of the pump for Na+ or H+ ions in a channel-like mode, again causing depolarization and release of [[aldosterone]].<ref name="pmid262526183">{{cite journal |vauthors=Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T |title=Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype |journal=Clin. Endocrinol. (Oxf) |volume=83 |issue=6 |pages=779–89 |year=2015 |pmid=26252618 |pmc=4995792 |doi=10.1111/cen.12873 |url=}}</ref>
| |
| '''<u>2. Familial hyperaldosteronism Type I (FH-I)</u>'''
| |
| *FH-I follows an [[Autosomal dominant inheritance|autosomal dominant]] inheritance pattern.<ref name="pmid11595502">{{cite journal |vauthors=Stowasser M, Gordon RD |title=Familial hyperaldosteronism |journal=J. Steroid Biochem. Mol. Biol. |volume=78 |issue=3 |pages=215–29 |year=2001 |pmid=11595502 |doi= |url= |issn=}}</ref><ref name="pmid12381543">{{cite journal |vauthors=Jackson RV, Lafferty A, Torpy DJ, Stratakis C |title=New genetic insights in familial hyperaldosteronism |journal=Ann. N. Y. Acad. Sci. |volume=970 |issue= |pages=77–88 |year=2002 |pmid=12381543 |doi= |url= |issn=}}</ref>
| |
| * Patients with FH-I inherit a [[Chimeric protein|chimeric]] ''[[CYP11B1]]'' and ''[[CYP11B2]]'' hybrid gene.<ref name="pmid11595502">{{cite journal |vauthors=Stowasser M, Gordon RD |title=Familial hyperaldosteronism |journal=J. Steroid Biochem. Mol. Biol. |volume=78 |issue=3 |pages=215–29 |year=2001 |pmid=11595502 |doi= |url= |issn=}}</ref>
| |
| '''<u>3. Familial hyperaldosteronism Type II (FH-II)</u>'''
| |
| *FH-II has an [[autosomal dominant inheritance]].<ref name="pmid12381543">{{cite journal |vauthors=Jackson RV, Lafferty A, Torpy DJ, Stratakis C |title=New genetic insights in familial hyperaldosteronism |journal=Ann. N. Y. Acad. Sci. |volume=970 |issue= |pages=77–88 |year=2002 |pmid=12381543 |doi= |url= |issn=}}</ref>
| |
| *It is caused due to [[Germline mutation|germline mutations]] on a locus on [[Chromosome 7 (human)|chromosome 7]] (specifically chromosome 7p22).<ref name="pmid11073536">{{cite journal |vauthors=Lafferty AR, Torpy DJ, Stowasser M, Taymans SE, Lin JP, Huggard P, Gordon RD, Stratakis CA |title=A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22) |journal=J. Med. Genet. |volume=37 |issue=11 |pages=831–5 |year=2000 |pmid=11073536 |pmc=1734468 |doi= |url= |issn=}}</ref><ref name="pmid9745430">{{cite journal |vauthors=Torpy DJ, Gordon RD, Lin JP, Huggard PR, Taymans SE, Stowasser M, Chrousos GP, Stratakis CA |title=Familial hyperaldosteronism type II: description of a large kindred and exclusion of the aldosterone synthase (CYP11B2) gene |journal=J. Clin. Endocrinol. Metab. |volume=83 |issue=9 |pages=3214–8 |year=1998 |pmid=9745430 |doi=10.1210/jcem.83.9.5086 |url= |issn=}}</ref>
| |
| '''<u>4. Familial hyperaldosteronism Type III</u>'''
| |
| *FH-III has been known to be caused due to mutation in the [[KCNJ5]] [[gene]].<ref name="urlGenetics of primary hyperaldosteronism">{{cite web |url=http://erc.endocrinology-journals.org/content/23/10/R437.long |title=Genetics of primary hyperaldosteronism |author= |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref>
| |
| *The [[tyrosine]]-to-[[cysteine]] substitution leads to increased Na(+) permeability, [[Depolarization|cell membrane depolarization]], and disturbed intracellular Ca(2+) homeostasis.<ref name="pmid24037882">{{cite journal |vauthors=Monticone S, Hattangady NG, Penton D, Isales CM, Edwards MA, Williams TA, Sterner C, Warth R, Mulatero P, Rainey WE |title=a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III |journal=J. Clin. Endocrinol. Metab. |volume=98 |issue=11 |pages=E1861–5 |year=2013 |pmid=24037882 |pmc=3816265 |doi=10.1210/jc.2013-2428 |url= |issn=}}</ref>
| |
| | |
| == Associated Conditions ==
| |
| The following conditions may be found in association with primary hyperaldosteronism:
| |
| * [[Anxiety]]
| |
| * [[Depression]]<ref name="pmid468762">{{cite journal |vauthors=Malinow KC, Lion JR |title=Hyperaldosteronism (Conn's disease) presenting as depression |journal=J Clin Psychiatry |volume=40 |issue=8 |pages=358–9 |year=1979 |pmid=468762 |doi= |url=}}</ref><ref name="pmid22568586">{{cite journal |vauthors=Apostolopoulou K, Künzel HE, Gerum S, Merkle K, Schulz S, Fischer E, Pallauf A, Brand V, Bidlingmaier M, Endres S, Beuschlein F, Reincke M |title=Gender differences in anxiety and depressive symptoms in patients with primary hyperaldosteronism: a cross-sectional study |journal=World J. Biol. Psychiatry |volume=15 |issue=1 |pages=26–35 |year=2014 |pmid=22568586 |doi=10.3109/15622975.2012.665480 |url=}}</ref>
| |
| * [[Crohn's disease]]<ref name="pmid15985978">{{cite journal |vauthors=Astegiano M, Bresso F, Demarchi B, Sapone N, Novero D, Palestro G, Resegotti A, Pellicano R, Rizzetto M |title=Association between Crohn's disease and Conn's syndrome. A report of two cases |journal=Panminerva Med |volume=47 |issue=1 |pages=61–4 |year=2005 |pmid=15985978 |doi= |url=}}</ref>
| |
| *[[Behçet's disease|Behcet's disease]]<ref name="pmid12589107">{{cite journal |vauthors=Kim YA, Lee SS |title=Conn's syndrome associated with Behcet's disease |journal=J. Korean Med. Sci. |volume=18 |issue=1 |pages=145–7 |year=2003 |pmid=12589107 |pmc=3054990 |doi=10.3346/jkms.2003.18.1.145 |url=}}</ref>
| |
| | |
| ==Gross Pathology==
| |
| * An [[aldosterone]] producing adenoma is usually, a unilateral, yellow, [[lipid]]-laden [[adenoma]] ranging in diameter from 5 to 35 mm.
| |
| [[Image:Gross_adrenal_adenoma.jpeg|200px|align right|Gross Pathology of Adrenal Adenoma]]
| |
| ==Microscopic Pathology==
| |
| Microscopically, on [[Hematoxylin and eosin stain|hematoxylin and eosin]] section the following findings can be observed for [[aldosterone]] producing [[Adenoma|adenomas]]:<ref name="urlAldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism">{{cite web |url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2889888/ |title=Aldosterone-producing adenoma and other surgically correctable forms of primary aldosteronism |author= |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=}}</ref>
| |
| * The [[tumor]] usually consists of [[zona fasciculata]]-type [[Cell (biology)|cells]] although [[zona glomerulosa]]- or mixed cell-type tumors have been described.
| |
| * [[Aldosterone]]-secreting [[Adrenal carcinoma|adrenal carcinomas]] are very rare. These [[malignant]] [[Tumor|tumors]] exceed 40 mm in size with invasion of local [[Lymph node|lymph nodes]] or invasion of adjacent organs.
| |
| [[Image:Adenoma_adrenal.jpg|200px|Microscopic Pathology of Adrenal Adenoma ]]
| |
|
| |
|
|
| |
|
| Line 83: |
Line 92: |
| ==References== | | ==References== |
| {{reflist|2}} | | {{reflist|2}} |
| {{WH}}
| |
| {{WS}}
| |
| [[Category:Endocrinology]]
| |
| [[Category:Needs content]]
| |