Trihexyphenidyl: Difference between revisions
No edit summary |
m (Protected "Trihexyphenidyl": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 64: | Line 64: | ||
* Tardive dyskinesia may appear in some patients on long-term therapy with antipsychotic drugs or may occur after therapy with these drugs has been discontinued. Antiparkinsonism agents do not alleviate the symptoms of tardive dyskinesia and, in some instances, may aggravate them. However, parkinsonism and tardive dyskinesia often coexist in patients receiving chronic neuroleptic treatment, and anticholinergic therapy with trihexyphenidyl HCl may relieve some of these parkinsonism symptoms. | * Tardive dyskinesia may appear in some patients on long-term therapy with antipsychotic drugs or may occur after therapy with these drugs has been discontinued. Antiparkinsonism agents do not alleviate the symptoms of tardive dyskinesia and, in some instances, may aggravate them. However, parkinsonism and tardive dyskinesia often coexist in patients receiving chronic neuroleptic treatment, and anticholinergic therapy with trihexyphenidyl HCl may relieve some of these parkinsonism symptoms. | ||
|clinicalTrials=Minor side effects, such as dryness of the mouth, blurring of vision, dizziness, mild nausea or nervousness, will be experienced by 30 to 50 percent of all patients. These sensations, however, are much less troublesome with trihexyphenidyl HCl than with belladonna alkaloids and are usually less disturbing than unalleviated parkinsonism. Such reactions tend to become less pronounced, and even to disappear, as treatment continues. Even before these reactions have remitted spontaneously, they may often be controlled by careful adjustment of dosage form, amount of drug, or interval between doses. | |clinicalTrials=* Minor side effects, such as dryness of the mouth, blurring of vision, dizziness, mild nausea or nervousness, will be experienced by 30 to 50 percent of all patients. These sensations, however, are much less troublesome with trihexyphenidyl HCl than with belladonna alkaloids and are usually less disturbing than unalleviated parkinsonism. Such reactions tend to become less pronounced, and even to disappear, as treatment continues. Even before these reactions have remitted spontaneously, they may often be controlled by careful adjustment of dosage form, amount of drug, or interval between doses. | ||
* Isolated instances of suppurative parotitis secondary to excessive dryness at the mouth, skin rashes, dilatation of the colon, paralytic ileus, and certain psychiatric manifestations such as delusions and hallucinations, plus one doubtful case of paranoia all of which may occur with any of the atropine-like drugs, have been reported rarely with trihexyphenidyl hydrochloride. | * Isolated instances of suppurative parotitis secondary to excessive dryness at the mouth, skin rashes, dilatation of the colon, paralytic ileus, and certain psychiatric manifestations such as delusions and hallucinations, plus one doubtful case of paranoia all of which may occur with any of the atropine-like drugs, have been reported rarely with trihexyphenidyl hydrochloride. | ||
| Line 124: | Line 124: | ||
|drugBox=[[File:Trihexyphenidyl wiki.png|600px|thumbnail|left]] | |drugBox=[[File:Trihexyphenidyl wiki.png|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
|mechAction=* Trihexyphenidyl exerts a direct inhibitory effect upon the parasympathetic nervous system. It also has a relaxing effect on smooth musculature; exerted both directly upon the muscle tissue itself and indirectly through an inhibitory effect upon the parasympathetic nervous system. Its therapeutic properties are similar to those of atropine, although undesirable side effects are ordinarily less frequent and severe than with the latter. | |mechAction=* Trihexyphenidyl exerts a direct inhibitory effect upon the parasympathetic nervous system. It also has a relaxing effect on smooth musculature; exerted both directly upon the muscle tissue itself and indirectly through an inhibitory effect upon the parasympathetic nervous system. Its therapeutic properties are similar to those of atropine, although undesirable side effects are ordinarily less frequent and severe than with the latter. | ||
| Line 134: | Line 133: | ||
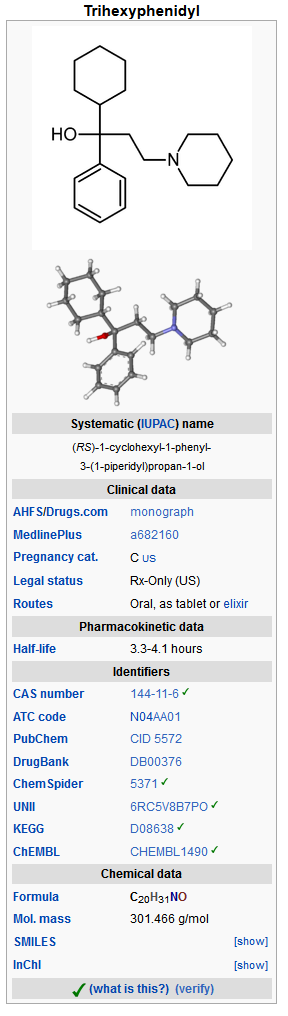
*Trihexyphenidyl HCl is the substituted piperidine salt, 1-piperidinepropanol,α-cyclohexyl-α-phenyl-,hydrochloride,(±)-. The structural formula is: | *Trihexyphenidyl HCl is the substituted piperidine salt, 1-piperidinepropanol,α-cyclohexyl-α-phenyl-,hydrochloride,(±)-. The structural formula is: | ||
[[File:Trihexyphenidyl structure. | [[File:Trihexyphenidyl structure.jpg|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
|PD=* There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=* There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
Latest revision as of 17:21, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Trihexyphenidyl is an anticholinergic, antiparkinson agent that is FDA approved for the treatment of parkinson's disease, parkinsonism due to drug. Common adverse reactions include nausea, xerostomia, dizziness, blurred vision, feeling nervous.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Parkinson's disease
- Dosing Information
- For treating PARKINSON'S DISEASE, an initial oral dose (tablet or elixir) of 1 milligram the first day followed by increases of 2 milligram increments at 3 to 5 day intervals to a total dose of 6 to 10 milligrams/day in 3 to 4 divided doses is recommended. Postencephalitic patients may require 12 to 15 milligrams/day.
- It is recommended that trihexyphenidyl be taken in 3 divided doses at mealtime. If the total daily dose is greater than 10 milligrams/day it may be given in 4 divided doses (at mealtime and bedtime).
- The recommended dose of trihexyphenidyl when used concomitantly with levodopa is 1 to 2 milligrams three times daily.
- Sustained release capsules should not be used for initial therapy due to the high dosage per capsule. After patients are stabilized on conventional therapy they may be switched to the sustained release form on a milligram for milligram basis as a single dose or 2 divided doses 12 hours apart.
Parkinsonism due to drug
- Dosing Information
- For treating DRUG-INDUCED PARKINSONISM, five to 15 milligrams as a total daily dose is recommended to control extrapyramidal effects from phenothiazines, thioxanthenes and butyrophenones. It is recommended that therapy be initiated with a single 1-milligram dose. If the extrapyramidal manifestations are not controlled in a few hours, subsequent doses may be progressively increased until satisfactory control is achieved.
- Satisfactory control may sometimes be more rapidly achieved by a temporary dose reduction of the tranquilizer when instituting trihexyphenidyl therapy and adjusting the dose of both drugs until the desired effect is obtained.
- Sustained release capsules should not be used for initial therapy due to the high dosage per capsule. After patients are stabilized on conventional therapy they may be switched to the sustained release form on a milligram for milligram basis as a single dose or 2 divided doses 12 hours apart.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Trihexyphenidyl in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Trihexyphenidyl in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Trihexyphenidyl in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Trihexyphenidyl in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Trihexyphenidyl in pediatric patients.
Contraindications
There is limited information regarding Trihexyphenidyl Contraindications in the drug label.
Warnings
- Patients to be treated with trihexyphenidyl HCl should have a gonioscope evaluation and close monitoring of intraocular pressures at regular periodic intervals.
PRECAUTIONS
- Although trihexyphenidyl HCl is not contraindicated for patients with cardiac, liver, or kidney disorders, or with hypertension, such patients should be maintained under close observation.
- Since the use of trihexyphenidyl HCl may, in some cases, continue indefinitely and since it has atropine-like properties, patients should be subjected to constant and careful long-term observation to avoid allergic and other untoward reactions. Inasmuch as trihexyphenidyl HCl possesses some parasympatholytic activity, it should be used with caution in patients with glaucoma, obstructive disease of the gastrointestinal or genitourinary tracts, and in elderly males with possible prostatic hypertrophy. Geriatric patients, particularly over the age of 60, frequently develop increased sensitivity to the actions of drugs of this type, and hence, require strict dosage regulation. Incipient glaucoma may be precipitated by parasympatholytic drugs such as trihexyphenidyl HCl.
- Tardive dyskinesia may appear in some patients on long-term therapy with antipsychotic drugs or may occur after therapy with these drugs has been discontinued. Antiparkinsonism agents do not alleviate the symptoms of tardive dyskinesia and, in some instances, may aggravate them. However, parkinsonism and tardive dyskinesia often coexist in patients receiving chronic neuroleptic treatment, and anticholinergic therapy with trihexyphenidyl HCl may relieve some of these parkinsonism symptoms.
Adverse Reactions
Clinical Trials Experience
- Minor side effects, such as dryness of the mouth, blurring of vision, dizziness, mild nausea or nervousness, will be experienced by 30 to 50 percent of all patients. These sensations, however, are much less troublesome with trihexyphenidyl HCl than with belladonna alkaloids and are usually less disturbing than unalleviated parkinsonism. Such reactions tend to become less pronounced, and even to disappear, as treatment continues. Even before these reactions have remitted spontaneously, they may often be controlled by careful adjustment of dosage form, amount of drug, or interval between doses.
- Isolated instances of suppurative parotitis secondary to excessive dryness at the mouth, skin rashes, dilatation of the colon, paralytic ileus, and certain psychiatric manifestations such as delusions and hallucinations, plus one doubtful case of paranoia all of which may occur with any of the atropine-like drugs, have been reported rarely with trihexyphenidyl hydrochloride.
- Patients with arteriosclerosis or with a history of idiosyncrasy to other drugs may exhibit reactions of mental confusion, agitation, disturbed behavior, or nausea and vomiting. Such patients should be allowed to develop a tolerance through the initial administration of a small dose and gradual increase in dose until an effective level is reached. If a severe reaction should occur, administration of the drug should be discontinued for a few days and then resumed at a lower dosage. Psychiatric disturbances can result from indiscriminate use (leading to overdosage) to sustain continued euphoria.
- Potential side effects associated with the use of any atropine-like drugs include constipation, drowsiness, urinary hesitancy or retention, tachycardia, dilation of the pupil, increased intraocular tension, weakness, vomiting, and headache.
- The occurrence of angle-closure glaucoma due to long-term treatment with trihexyphenidyl has been reported.
Postmarketing Experience
There is limited information regarding Trihexyphenidyl Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Trihexyphenidyl Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trihexyphenidyl in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Trihexyphenidyl during labor and delivery.
Nursing Mothers
- There is no FDA guidance on the use of Trihexyphenidyl with respect to nursing mothers.
Pediatric Use
- There is no FDA guidance on the use of Trihexyphenidyl with respect to pediatric patients.
Geriatic Use
- There is no FDA guidance on the use of Trihexyphenidyl with respect to geriatric patients.
Gender
- There is no FDA guidance on the use of Trihexyphenidyl with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Trihexyphenidyl with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Trihexyphenidyl in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Trihexyphenidyl in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Trihexyphenidyl in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Trihexyphenidyl in patients who are immunocompromised.
Administration and Monitoring
Administration
- Dosage should be individualized. The initial dose should be low and then increased gradually, especially in patients over 60 years of age. Whether trihexyphenidyl HCl may best be given before or after meals should be determined by the way the patient reacts. Postencephalitic patients, who are usually more prone to excessive salivation, may prefer to take it after meals and may, in addition, require small amounts of atropine which, under such circumstances, is sometimes an effective adjuvant. If trihexyphenidyl HCl tends to dry the mouth excessively, it may be better to take it before meals, unless it causes nausea. If taken after meals, the thirst sometimes induced can be allayed by mint candies, chewing gum or water.
- As initial therapy for parkinsonism, 1 mg of trihexyphenidyl in tablet form may be administered the first day. The dose may then be increased by 2 mg increments at intervals of three to five days, until a total of 6 to 10 mg is given daily. The total daily dose will depend upon what is found to be the optimal level. Many patients derive maximum benefit from this daily total of 6 to 10 mg, but some patients, chiefly those in the postencephalitic group, may require a total daily dose of 12 to 15 mg.
- The size and frequency of dose of trihexyphenidyl HCl needed to control extrapyramidal reactions to commonly employed tranquilizers, notably the phenothiazines, thioxanthenes, and butyrophenones, must be determined empirically. The total daily dosage usually ranges between 5 and 15 mg, although, in some cases, these reactions have been satisfactorily controlled on as little as 1 mg daily. It may be advisable to commence therapy with a single 1 mg dose. If the extrapyramidal manifestations are not controlled in a few hours, the subsequent doses may be progressively increased until satisfactory control is achieved. Satisfactory control may sometimes be more rapidly achieved by temporarily reducing the dosage of the tranquilizer on instituting trihexyphenidyl HCl therapy and then adjusting dosage of both drugs until the desired ataractic effect is retained without onset of extrapyramidal reactions.
- It is sometimes possible to maintain the patient on a reduced trihexyphenidyl HCl dosage after the reactions have remained under control for several days. Instances have been reported in which these reactions have remained in remission for long periods after trihexyphenidyl HCl therapy was discontinued.
- When trihexyphenidyl HCl is used concomitantly with levodopa, the usual dose of each may need to be reduced. Careful adjustment is necessary, depending on side effects and degree of symptom control. Trihexyphenidyl HCl dosage of 3 to 6 mg daily, in divided doses, is usually adequate.
- Trihexyphenidyl HCl may be substituted, in whole or in part, for other parasympathetic inhibitors. The usual technique is partial substitution initially, with progressive reduction in the other medication as the dose of trihexyphenidyl HCl is increased.
- The total daily intake of trihexyphenidyl HCl tablets is tolerated best if divided into 3 doses and taken at mealtimes. High doses (>10 mg daily) may be divided into 4 parts, with 3 doses administered at mealtimes and the fourth at bedtime.
Monitoring
- There is limited information regarding Monitoring of Trihexyphenidyl in the drug label.
IV Compatibility
- There is limited information regarding IV Compatibility of Trihexyphenidyl in the drug label.
Overdosage
- There is limited information regarding Chronic Overdose of Trihexyphenidyl in the drug label.
Pharmacology
Mechanism of Action
- Trihexyphenidyl exerts a direct inhibitory effect upon the parasympathetic nervous system. It also has a relaxing effect on smooth musculature; exerted both directly upon the muscle tissue itself and indirectly through an inhibitory effect upon the parasympathetic nervous system. Its therapeutic properties are similar to those of atropine, although undesirable side effects are ordinarily less frequent and severe than with the latter.
Structure
- Trihexyphenidyl HCl is a synthetic antispasmodic. Each tablet for oral administration, contains 2 mg or 5 mg trihexyphenidyl HCl, each strength also containing as inactive ingredients: magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
- Trihexyphenidyl HCl is a white or slightly off white, crystalline powder, having not more than a very faint odor.
- Trihexyphenidyl HCl is the substituted piperidine salt, 1-piperidinepropanol,α-cyclohexyl-α-phenyl-,hydrochloride,(±)-. The structural formula is:

Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Trihexyphenidyl in the drug label.
Pharmacokinetics
- There is limited information regarding Pharmacokinetics of Trihexyphenidyl in the drug label.
Nonclinical Toxicology
- There is limited information regarding Nonclinical Toxicology of Trihexyphenidyl in the drug label.
Clinical Studies
- There is limited information regarding Clinical Studies of Trihexyphenidyl in the drug label.
How Supplied
- Trihexyphenidyl HCl tablets are available as follows:
- 2 mg – round, flat, scored, white tablets; debossed "5971" above the score and "V" below the score. Available in bottles of 100, 500 and 1000.
- 5 mg – round, flat, scored, white tablets; debossed "5972" above the score and "V" below the score. Available in bottles of 100, 500, and 1000.
Storage
- Dispense in a tight, light-resistant container as defined in the USP.
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Trihexyphenidyl |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Trihexyphenidyl |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- There is limited information regarding Patient Counseling Information of Trihexyphenidyl in the drug label.
Precautions with Alcohol
- Alcohol-Trihexyphenidyl interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Artane, Trihexane, Tritane.
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Trihexyphenidyl
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Trihexyphenidyl |Label Name=Trihexyphenidyl11.png
}}
{{#subobject:
|Label Page=Trihexyphenidyl |Label Name=Trihexyphenidyl11.png
}}