Zidovudine description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Description
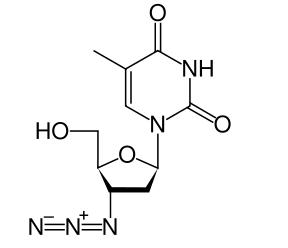
RETROVIR is the brand name for zidovudine (formerly called azidothymidine [AZT]), a pyrimidine nucleoside analogue active against HIV-1. The chemical name of zidovudine is 3′-azido-3′-deoxythymidine; it has the following structural formula:
 |
 |
 |
Zidovudine is a white to beige, odorless, crystalline solid with a molecular weight of 267.24 and a solubility of 20.1 mg/mL in water at 25°C. The molecular formula is C10H13N5O4.
RETROVIR Tablets are for oral administration. Each film-coated tablet contains 300 mg of zidovudine and the inactive ingredients hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium starch glycolate, and titanium dioxide.
RETROVIR Capsules are for oral administration. Each capsule contains 100 mg of zidovudine and the inactive ingredients corn starch, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate. The 100-mg empty hard gelatin capsule, printed with edible black ink, consists of black iron oxide, dimethylpolysiloxane, gelatin, pharmaceutical shellac, soya lecithin, and titanium dioxide. RETROVIR Syrup is for oral administration. Each mL of RETROVIR Syrup contains 10 mg of zidovudine and the inactive ingredients sodium benzoate 0.2% (added as a preservative), citric acid, flavors, glycerin, and liquid sucrose. Sodium hydroxide may be added to adjust pH.[1]
References
Adapted from the FDA Package Insert.