Triamcinolone acetonide (Zilretta)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Triamcinolone acetonide (Zilretta) is a extended-release synthetic corticosteroid that is FDA approved for the management of osteoarthritis pain of the knee. Common adverse reactions include sinusitis, cough, and contusions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Triamcinolone acetonide extended-release injectable suspension is indicated as an intra-articular injection for the management of osteoarthritis pain of the knee.
Limitation of Use
- Triamcinolone acetonide is not intended for repeat administration.
Important Dosage and Administration Information
- Triamcinolone acetonide is administered as a single intra-articular extended-release injection of triamcinolone acetonide, to deliver 32 mg (5 mL).
- Triamcinolone acetonide is for intra-articular use only and should not be administered by the following routes: epidural, intrathecal, intravenous, intraocular, intramuscular, intradermal, subcutaneous.
- Triamcinolone acetonide is not suitable for use in small joints, such as the hand.
- The efficacy and safety of repeat administration of triamcinolone acetonide for the management of osteoarthritis pain of the knee have not been evaluated.
- The efficacy and safety of triamcinolone acetonide for management of osteoarthritis pain of shoulder and hip have not been evaluated.
Non-Interchangeability with Other Formulations of Triamcinolone Acetonide for Intra-articular Use
- Triamcinolone acetonide is not interchangeable with other formulations of injectable triamcinolone acetonide.
Dosage Forms and Strengths
- Triamcinolone acetonide is an injectable suspension that delivers 32 mg of triamcinolone acetonide. Triamcinolone acetonide is supplied as a single-dose kit, containing:
- One vial of triamcinolone acetonide white to off-white microsphere powder
- One vial of 5 mL sterile, clear diluent
- One sterile vial adapter
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding triamcinolone acetonide Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding triamcinolone acetonide Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Triamcinolone acetonide (Zilretta) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding triamcinolone acetonide Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding triamcinolone acetonide Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Triamcinolone acetonide is contraindicated in patients who are hypersensitive to triamcinolone acetonide, corticosteroids or any components of the product.
Warnings
Warnings and Precautions Specific for Triamcinolone Acetonide
- Triamcinolone acetonide has not been evaluated and should not be administered by the following routes:
- Epidural
- Intrathecal
- Intravenous
- Intraocular
- Intramuscular
- Intradermal
- Subcutaneous
Serious Neurologic Adverse Reactions with Epidural and Intrathecal Administration
- Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids. Specific events reported include, but are not limited to, spinal cord infarction, paraplegia, quadriplegia, cortical blindness, and stroke. These serious neurologic events have been reported with and without use of fluoroscopy.
- Reports of serious medical events have been associated with the intrathecal route of corticosteroid administration.
- The safety and effectiveness of epidural and intrathecal administration of corticosteroids have not been established, and corticosteroids are not approved for this use. In particular, the formulation of triamcinolone acetonide should not be considered safe to use for epidural or intrathecal administration.
Hypersensitivity Reactions
- Rare instances of anaphylaxis have occurred in patients with hypersensitivity to corticosteroids. Cases of serious anaphylaxis, including death, have been reported in individuals receiving triamcinolone acetonide injection, regardless of the route of administration. Institute appropriate care upon occurrence of an anaphylactic reaction.
Joint Infection and Damage
- Intra-articular injection of corticosteroid may be complicated by joint infection. A marked increase in pain accompanied by local swelling, further restriction of joint motion, fever, and malaise are suggestive of septic arthritis. If this complication occurs and a diagnosis of septic arthritis is confirmed, institute appropriate antimicrobial therapy.
- Avoid injection of a corticosteroid into an infected site. Local injection of a corticosteroid into a previously infected joint is not usually recommended. Examine any joint fluid present to exclude a septic process.
- Corticosteroid injection into unstable joints is generally not recommended.
- Intra-articular injection may result in damage to joint tissues.
Increased Risk of Infections
- Intra-articularly injected corticosteroids are systemically absorbed. Patients who are on corticosteroids are more susceptible to infections than are healthy individuals. There may be decreased resistance and inability to localize infection when corticosteroids are used. Infection with any pathogen (viral, bacterial, fungal, protozoan, or helminthic) in any location of the body may be associated with the use of corticosteroids alone or in combination with other immunosuppressive agents. These infections may be mild to severe. With increasing doses of corticosteroids, the rate of occurrence of infectious complications increases. Corticosteroids may also mask some signs of current infection.
- Advise patients to inform their health care provider if they develop fever or other signs or symptoms of infection. Advise patients who have not been vaccinated to avoid exposure to chicken pox or measles. Instruct patients to contact their health care provider immediately if they are exposed.
Alterations in Endocrine Function
- Corticosteroids can produce reversible hypothalamic-pituitary-adrenal axis suppression, with the potential for adrenal insufficiency after withdrawal of treatment, which may persist for months.
- In situations of stress during that period (as in trauma, surgery, or illness), institute corticosteroid replacement therapy.
- Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients.
Cardiovascular Effects
- Corticosteroids can cause elevations of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with synthetic derivatives.
- Monitor patients with congestive heart failure or hypertension for signs of edema, weight gain, and imbalance in serum electrolytes. Dietary salt restriction and potassium supplementation may be necessary.
Renal Effects
- Corticosteroids can cause salt and water retention, and increased excretion of potassium. These effects are less likely to occur with synthetic derivatives. All corticosteroids increase calcium excretion.
- Monitor patients with renal insufficiency for signs of edema, weight gain, and imbalance in serum electrolytes. Dietary salt restriction and potassium supplementation may be necessary.
Increased Intraocular Pressure
- Corticosteroid use may be associated with development or exacerbation of increased intraocular pressure.
- Monitor patients with elevated intraocular pressure for potential treatment adjustment.
Gastrointestinal Perforation
- Corticosteroid administration is associated with increased risk of gastrointestinal perforation in patients with certain GI disorders such as active or latent peptic ulcers, diverticulosis, diverticulitis, ulcerative colitis and in patients with fresh intestinal anastomoses.
- Avoid corticosteroids in these patients because signs of peritoneal irritation following gastrointestinal perforation may be minimal or absent.
Alterations in Bone Density
- Corticosteroids decrease bone formation and increase bone resorption through their effect on calcium regulation and inhibition of osteoblast function.
- Special consideration should be given to patients with or at increased risk of osteoporosis (e.g., postmenopausal women) before initiating corticosteroid therapy.
Behavioral and Mood Disturbances
- Corticosteroid use may be associated with new or aggravated adverse psychiatric reactions ranging from euphoria, insomnia, mood swings, and personality changes to severe depression and frank psychotic manifestations.
- Special consideration should be given to patients with previous or current emotional instability or psychiatric illness before initiating corticosteroid therapy. Advise patients and/or caregivers to immediately report any new or worsening behavior or mood disturbances to their health care provider.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data below reflect exposure to a single 32 mg intra-articular injection of triamcinolone acetonide in clinical studies in patients with moderate to severe pain due to osteoarthritis of the knee. Clinical studies included randomized, double-blind, parallel-group, placebo and/or active-controlled, and pharmacokinetic/pharmacodynamic studies with follow-up ranging from 6-24 weeks. A total of 424 patients received triamcinolone acetonide and 262 received placebo. Treatment emergent adverse reactions reported by greater than or equal to 1% of patients in the triamcinolone acetonide arms are summarized below ( TABLE 1 and 2).
- Overall, the incidence and nature of adverse reactions was similar to that observed with placebo.

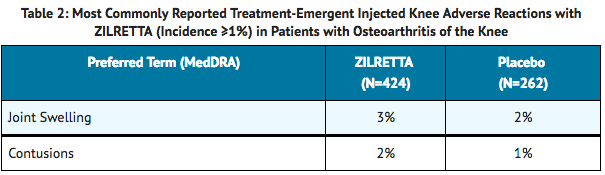
Corticosteroid Adverse Reactions
- The following adverse reactions, presented alphabetically by body system, are from voluntary reports or clinical studies of corticosteroids. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylactic reactions
- Anaphylaxis including death, angioedema
Cardiovascular
- Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, hypertension, fat embolism, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction, pulmonary edema, syncope, tachycardia, thromboembolism, thrombophlebitis, vasculitis.
Dermatologic
- Acne, allergic dermatitis, cutaneous and subcutaneous atrophy, dry scaly skin, ecchymoses and petechiae, edema, erythema, hyperpigmentation, hypopigmentation, impaired wound healing, increased sweating, lupus erythematosus-like lesions, purpura, rash, sterile abscess, striae, suppressed reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria.
Endocrine
- Decreased carbohydrate and glucose tolerance, development of Cushingoid state, glycosuria, hirsutism, hypertrichosis, increased requirements for insulin or oral hypoglycemic agents in diabetes, manifestations of latent diabetes mellitus, menstrual irregularities, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery, or illness), suppression of growth in pediatric patients.
Fluid and electrolyte disturbances
- Congestive heart failure in susceptible patients, fluid retention, sodium retention.
Gastrointestinal
- Abdominal distention, bowel/bladder dysfunction (after intrathecal administration), elevation in serum liver enzyme levels (usually reversible upon discontinuation), hepatomegaly, increased appetite, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, perforation of the small and large intestine (particularly in patients with inflammatory bowel disease), ulcerative esophagitis.
Metabolic
- Negative nitrogen balance due to protein catabolism.
Musculoskeletal
- Aseptic necrosis of femoral and humeral heads, calcinosis (following intra-articular or intralesional use), Charcot-like arthropathy, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, post injection flare (following intra-articular use), steroid myopathy, tendon rupture, vertebral compression fractures.
Neurologic/Psychiatric
- Convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudotumor cerebri) usually following discontinuation of treatment, insomnia, mood swings, neuritis, neuropathy, paresthesia, personality changes, psychiatric disorders, vertigo. Arachnoiditis, meningitis, paraparesis/paraplegia, and sensory disturbances have occurred after intrathecal administration. Spinal cord infarction, paraplegia, quadriplegia, cortical blindness, and stroke (including brainstem) have been reported after epidural administration of corticosteroids.
Ophthalmic
- Exophthalmos, glaucoma, increased intraocular pressure, posterior subcapsular cataracts, rare instances of blindness associated with periocular injections.
Other
- Abnormal fat deposits, decreased resistance to infection, hiccups, increased or decreased motility and number of spermatozoa, malaise, moon face, weight gain.
Postmarketing Experience
There is limited information regarding Triamcinolone acetonide (Zilretta) Postmarketing Experience in the drug label.
Drug Interactions
- No drug-drug interaction studies have been conducted with triamcinolone acetonide. TABLE 3 contains drug interactions associated with systemic corticosteroids.

Use in Specific Populations
Pregnancy
Risk Summary
- There are no data regarding the use of triamcinolone acetonide in pregnant women to inform a drug associated risk of adverse developmental outcomes. Published studies on the association between corticosteroids and fetal outcomes have reported inconsistent findings and have important methodological limitations. The majority of published literature with corticosteroid exposure during pregnancy includes the oral, topical and inhaled dosage formulations; therefore, the applicability of these findings to a single intra-articular injection of triamcinolone acetonide is limited. In animal reproductive studies from the published literature, pregnant mice, rats, rabbits, or primates administered triamcinolone acetonide during the period of organogenesis at doses that produced exposures less than the maximum recommended human dose (MRHD) caused resorptions, decreased fetal body weight, craniofacial and/or other abnormalities such as omphalocele.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data (Animal)
- The exposure margins listed below are based on body surface area comparisons (mg/m 2) to the highest daily triamcinolone acetonide exposure at the MRHD of 32 mg triamcinolone acetonide via triamcinolone acetonide.
- Pregnant mice dosed with triamcinolone acetonide via intramuscular or subcutaneous injection at doses equivalent to 0.8 times the MRHD or higher during organogenesis caused cleft palate and a higher rate of resorption. In pregnant rats dosed with triamcinolone acetonide via intramuscular or subcutaneous injection at doses equivalent to 0.3 times the MRHD or higher during organogenesis caused developmental abnormality (cleft palate, omphalocele, late resorption, and growth retardation) and fetal mortality. No notable maternal toxicity was observed in rodents.
- Pregnant rabbits dosed with triamcinolone acetonide via intramuscular injection for 4 days during organogenesis at doses equivalent to 0.15 times the MRHD or higher caused resorption and cleft palate. No notable maternal toxicity was observed.
- Pregnant primates dosed with triamcinolone acetonide via intramuscular injection for 4 days during organogenesis at doses equivalent to 3 times the MRHD or higher caused severe craniofacial CNS and skeletal/visceral malformation and higher prenatal death. No notable maternal toxicity was observed.
- No peri- and post-natal development studies of triamcinolone acetonide in animals have been conducted.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Triamcinolone acetonide (Zilretta) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Triamcinolone acetonide (Zilretta) during labor and delivery.
Nursing Mothers
Risk Summary
- There are no available data on the presence of triamcinolone acetonide in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. However, corticosteroids have been detected in human milk and may suppress milk production. It is not known whether intra-articular administration of triamcinolone acetonide could result in sufficient systemic absorption to produce detectable quantities in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for triamcinolone acetonide and any potential adverse effects on the breastfed infant from triamcinolone acetonide or from the underlying maternal condition.
Pediatric Use
- The safety and effectiveness of triamcinolone acetonide in pediatric patients have not been established.
- The adverse effects of corticosteroids in pediatric patients are similar to those in adults. Carefully observe pediatric patients, including weight, height, linear growth, blood pressure, intraocular pressure, and clinical evaluation for the presence of infection, psychosocial disturbances, thromboembolism, peptic ulcers, cataracts, and osteoporosis. Weigh potential growth effects of treatment against clinical benefits obtained and the availability of treatment alternatives.
Geriatic Use
- Of the total number of patients administered 32 mg triamcinolone acetonide in clinical studies (N=424), 143 patients were 65 years of age or older. No overall differences in safety or effectiveness were observed between elderly and younger subjects, and other reported clinical experience with triamcinolone acetonide has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Triamcinolone acetonide (Zilretta) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Triamcinolone acetonide (Zilretta) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Triamcinolone acetonide (Zilretta) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Triamcinolone acetonide (Zilretta) in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Corticosteroids may result in menstrual pattern irregularities such as deviations in timing and duration of menses and an increased or decreased loss of blood.
Immunocompromised Patients
There is no FDA guidance one the use of Triamcinolone acetonide (Zilretta) in patients who are immunocompromised.
Administration and Monitoring
Administration
Important Dosage and Administration Information
- Triamcinolone acetonide is administered as a single intra-articular extended-release injection of triamcinolone acetonide, to deliver 32 mg (5 mL).
- Triamcinolone acetonide is for intra-articular use only and should not be administered by the following routes: epidural, intrathecal, intravenous, intraocular, intramuscular, intradermal, subcutaneous.
- Triamcinolone acetonide is not suitable for use in small joints, such as the hand.
- The efficacy and safety of repeat administration of triamcinolone acetonide for the management of osteoarthritis pain of the knee have not been evaluated.
- The efficacy and safety of triamcinolone acetonide for management of osteoarthritis pain of shoulder and hip have not been evaluated.
Preparation and Administration of Intra-Articular Suspension
- Triamcinolone acetonide is supplied as a single-dose kit containing a vial of triamcinolone acetonide microsphere powder, a vial of sterile diluent, and a sterile vial adapter.
- Triamcinolone acetonide must be prepared using the diluent supplied in the kit.
- Preparation of triamcinolone acetonide requires close attention to the INSTRUCTIONS FOR USE to ensure successful administration.
- Use proper aseptic technique throughout the dose preparation and administration procedure.
- Triamcinolone acetonide is a suspension product and it is normal for some residue to be left behind on the vial walls after withdrawing the contents.
- Promptly inject triamcinolone acetonide after preparation to avoid settling of the suspension. If needed, the triamcinolone acetonide suspension can be stored in the vial for up to 4 hours at ambient conditions. Gently swirl the vial to resuspend any of the settled microspheres prior to preparing the syringe for injection.
- The usual technique for intra-articular injection should be followed. Aspiration of synovial fluid may be performed based on clinical judgment prior to administration of triamcinolone acetonide.
Monitoring
- Improvement in the signs and symptoms of osteoarthritis of the knee-related pain may be indicative of efficacy.
- Imbalance in serum electrolytes in patients with congestive heart failure, hypertension, or renal insufficiency.
- Edema or weight gain in patients with congestive heart failure, hypertension, or renal insufficiency.
- Elevated intraocular pressure.
IV Compatibility
There is limited information regarding the compatibility of Triamcinolone acetonide (Zilretta) and IV administrations.
Overdosage
There is limited information regarding Triamcinolone acetonide (Zilretta) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Triamcinolone acetonide (Zilretta)
| |
| Systematic (IUPAC) name | |
| (4aS,4bR,5S,6aS,6bS,9aR,10aS,10bS)-4b-fluoro-6b-glycoloyl-5-hydroxy-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 434.504 g/mol |
| Synonyms | 9α-Fluoro-16α-17α-isopropyledenedioxyprednisolone; 9α-Fluoro-16α-hydroxyprednisolone 16α,17α-acetonide; 9α-Fluoro-11β,16α-17α,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone; 9α-Fluoro-11β,21-dihydroxy-16α,17α-isopropylidenedioxypregna-1,4-diene,3,20-dione |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Topical, joint injection |
Mechanism of Action
- Triamcinolone acetonide is a corticosteroid with anti-inflammatory and immunomodulating properties. It binds to and activates the glucocorticoid receptor, leading to activation of anti-inflammatory transcription factors such as lipocortins and inhibition of inflammatory transduction pathways by blocking the release of arachidonic acid and preventing the synthesis of prostaglandins and leukotrienes.
Structure

Pharmacodynamics
- Studies indicate that following a single intramuscular dose of 60 to 100 mg of immediate-release triamcinolone acetonide injectable suspension, adrenal suppression occurs within 24 to 48 hours and then gradually returns to normal, usually in 30 to 40 days. To assess potential effects of the systemic levels of triamcinolone acetonide associated with a single intra-articular (IA) administration of triamcinolone acetonide on hypothalamic pituitary adrenal (HPA) axis function, serum and urine cortisol levels were monitored over 6 weeks post injection. Adrenal suppression with triamcinolone acetonide occurred within 12-24 hours and then gradually returned to normal, within 30-42 days.
- Corticosteroids may increase blood glucose concentrations.
- In a study where 18 patients with osteoarthritis knee pain and controlled type 2 diabetes mellitus received a single IA injection of triamcinolone acetonide into the knee, the change from baseline in average blood glucose over the 72 hours after injection as measured by a continuous glucose monitoring device was 8.2 mg/dL (95% confidence interval 0.1, 29.2).
Pharmacokinetics
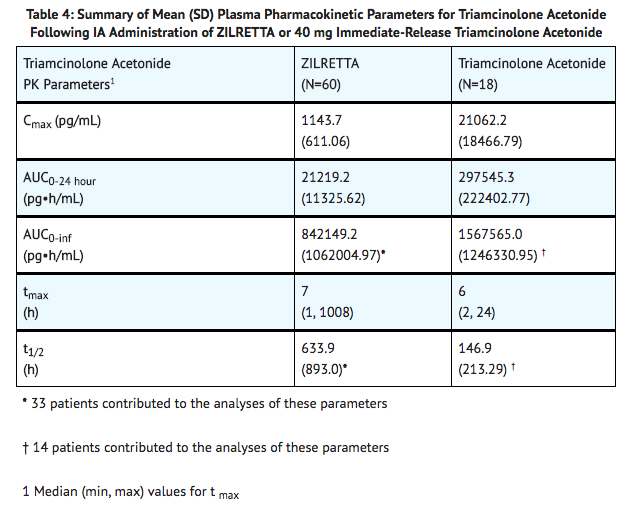
- Triamcinolone acetonide is an extended-release dosage form consisting of microspheres of poly(lactic-co-glycolic acid) (PLGA) containing triamcinolone acetonide. Plasma pharmacokinetic parameters for triamcinolone acetonide following IA administration of triamcinolone acetonide or 40 mg immediate-release triamcinolone acetonide into the knee are provided in TABLE 4.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Long-term animal studies to evaluate the carcinogenic potential of triamcinolone acetonide have not been conducted.
Mutagenesis
- Adequate mutagenicity studies have not been conducted with triamcinolone acetonide.
Impairment of Fertility
- Studies in animals to evaluate the impairment of fertility of triamcinolone acetonide have not been conducted.
Clinical Studies
- The efficacy of triamcinolone acetonide was demonstrated in a multi-center, international, randomized, double-blind, parallel-arm, placebo- and active-controlled study in patients with osteoarthritis pain of the knee. A total of 484 patients (triamcinolone acetonide 32 mg, N=161; placebo [saline], N=162; active control [a crystalline suspension, immediate-release formulation of triamcinolone acetonide 40 mg], N=161) were treated and followed for up to 24 weeks. Patients had a mean age of 62 (range 40 to 85 years); baseline demographics and disease characteristics were balanced across treatment arms. Twenty-five percent (25%) of patients had received at least one prior corticosteroid intra-articular injection more than 3 months prior to treatment. A total of 470 patients (97%) completed follow-up to Week 12, the time point for primary efficacy determination, and 443 (91.5%) completed to Week 24.
- The primary efficacy endpoint comparing triamcinolone acetonide to placebo was change from baseline at Week 12 in the weekly mean of the Average Daily Pain intensity scores (ADP) as assessed by a 0-10 Numeric Rating Scale (NRS). Triamcinolone acetonide demonstrated a statistically significant reduction in pain intensity at the primary endpoint vs placebo. Triamcinolone acetonide also demonstrated a reduction in pain intensity scores each week from Weeks 1 through 12 ( FIGURE 1).
- In a secondary exploratory analysis, statistical significance was not demonstrated between the triamcinolone acetonide and the active control (immediate-release triamcinolone acetonide) treatment groups for the change from baseline at Week 12 in weekly mean ADP.

How Supplied

Storage
- To maintain expiry period, refrigerate the triamcinolone acetonide single-dose kit (36°-46°F; 2°-8°C) before use.
- If refrigeration is unavailable, store the triamcinolone acetonide single-dose kit in the sealed, unopened kit at temperatures not exceeding 77°F (25°C) for up to six weeks and then discard. Do not expose the triamcinolone acetonide single-dose kit to temperatures above 77°F (25°C).
- Do not freeze. Store vials in carton.
Images
Drug Images
{{#ask: Page Name::Triamcinolone acetonide (Zilretta) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel






{{#ask: Label Page::Triamcinolone acetonide (Zilretta) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Increased Risk of Infections
- Inform patients that they may be more likely to develop infections when taking corticosteroids. Instruct patients to contact their health care provider if they develop fever or other signs or symptoms of infection.
- Advise patients who have not been vaccinated to avoid exposure to chicken pox or measles. Instruct patients to contact their health care provider immediately if they are exposed.
Risk of Drug Interactions
- There are a number of medicines that can interact with corticosteroids such as triamcinolone acetonide. Advise patients to alert their health care provider(s) to assess the need to adjust their medication(s).
Risk of Adverse Psychiatric Reactions
- Inform patients that corticosteroid use may be associated with adverse psychiatric reactions. Advise patients and/or caregivers to immediately report any new or worsening behavioral or mood disturbances to their health care provider.

Precautions with Alcohol
Alcohol-Triamcinolone acetonide (Zilretta) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Zilretta
Look-Alike Drug Names
There is limited information regarding Triamcinolone acetonide (Zilretta) Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.