Tonicity
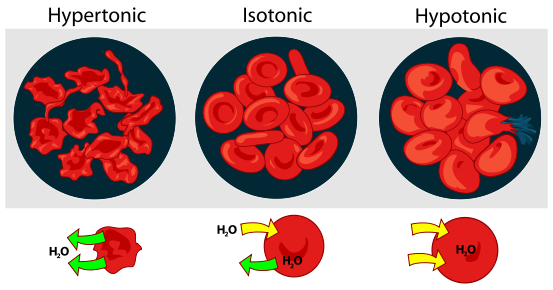
Tonicity is a measure of effective osmolarity or effective osmolality in cell biology. Osmolality and osmolarity are properties of a particular solution, independent of any membrane. Tonicity is a property of a solution in reference to a particular membrane, and is equal to the sum of the concentrations of the solutes which have the capacity to exert an osmotic force across that membrane. Tonicity, also, depends on solute permeability (permeant solutes do not affect tonicity; impermeant solutes do affect tonicity). Tonicity is generally classified in three ranges; hypertonicity, hypotonicity and isotonicity.
Hypertonicity
A cell in a hypertonic environment is surrounded by a higher concentration of impermeable solute than exists in the inside of the cell. Osmotic pressure directs a net amount of water out of the cell, causing it to shrink. Hypertonic, isotonic and hypotonic solutions are defined in reference to a cell membrane by comparing the tonicity of the solution with the tonicity within the cell.
In animal cells, being in a hypertonic environment results in crenation, where the shape of the cell becomes distorted and wrinkled as water leaves the cell. Some organisms have evolved methods of circumventing hypertonicity; for example, saltwater is hypertonic to the fish that live in it. Since they cannot isolate themselves from osmotic water loss, because they need a large surface area in their gills for gas exchange, they respond by drinking large amounts of water, and excreting the salt. This process is called osmoregulation.
In plant cells, the effect is more dramatic. The cell membrane pulls away from the cell wall, but the cell remains joined to the adjacent cells at points called plasmodesmata. Thus, the cell takes on the appearance of a pincushion, with the plasmodesmata almost ceasing to function because they have become so constricted. This condition is known as plasmolysis.
Isotonicity
A cell in an isotonic environment is in a state of equilibrium with its surroundings. When the amount of impermeable solute is the same on the inside and outside of the cell, osmotic pressure becomes equal; the force of water trying to exit and enter the cell balances out. This pressure is what drives hypertonic or hypotonic cells to become isotonic.
Hypotonicity
The opposite of a hypertonic environment is a hypotonic one, where the net movement of water is into the cell. If the cell contains more impermeable solute than its surroundings, water will enter it. In the case of animal cells, they will swell until they burst; plant cells do not burst, due to the reinforcement their cell wall provides.
See also
| Stub icon | This science article is a stub. You can help Wikipedia by expanding it. |
ka:ჰიპერტონიული ხსნარი nl:Hypertoniciteit simple:Hypertonic sv:Hyperton