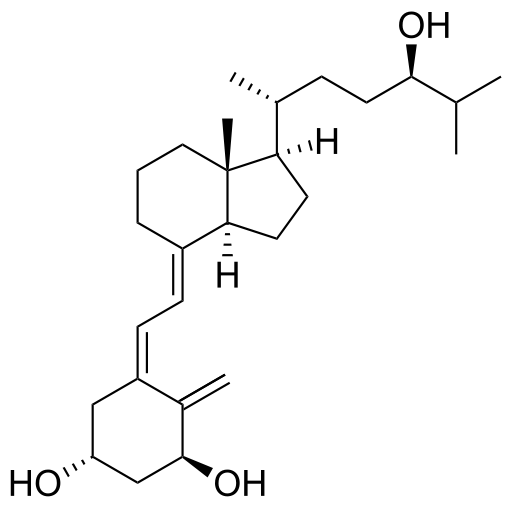
Tacalcitol
 | |
| Clinical data | |
|---|---|
| Synonyms | (1α,24R)-1,24-Dihydroxyvitamin D3 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Topical |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C27H44O3 |
| Molar mass | 416.64 |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Tacalcitol |
|
Articles |
|---|
|
Most recent articles on Tacalcitol |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Tacalcitol at Clinical Trials.gov Clinical Trials on Tacalcitol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Tacalcitol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Tacalcitol Discussion groups on Tacalcitol Patient Handouts on Tacalcitol Directions to Hospitals Treating Tacalcitol Risk calculators and risk factors for Tacalcitol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Tacalcitol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Tacalcitol (1,24-dihydroxyvitamin D3) is a synthetic vitamin D3 analog.[1]
Tacalcitol is marketed under several names, including Curatoderm and Bonalfa.
Mechanism
Tacalcitol reduces excessive cell turnover in the epidermis by interacting with vitamin D receptors on keratinocytes.
Uses
It is usually prescribed by a general practitioner or dermatologist for the treatment of psoriasis, chronic chapped lips and other severe dry skin conditions because of its ability to reduce excessive skin cell turnover. It is available as an ointment or lotion.
It has also been used for vitiligo[2] and Hailey-Hailey disease.[3]
References
- ↑ Fukuoka M, Sakurai K, Ohta T, Kiyoki M, Katayama I (2001). "Tacalcitol, an active vitamin D3, induces nerve growth factor production in human epidermal keratinocytes". Skin Pharmacol. Appl. Skin Physiol. 14 (4): 226–33. doi:10.1159/000056351. PMID 11464105.
- ↑ Leone G, Pacifico A, Iacovelli P, Paro Vidolin A, Picardo M (March 2006). "Tacalcitol and narrow-band phototherapy in patients with vitiligo". Clin. Exp. Dermatol. 31 (2): 200–5. doi:10.1111/j.1365-2230.2005.02037.x. PMID 16487090.
- ↑ Aoki T, Hashimoto H, Koseki S, Hozumi Y, Kondo S (November 1998). "1alpha,24-dihydroxyvitamin D3 (tacalcitol) is effective against Hailey-Hailey disease both in vivo and in vitro". Br. J. Dermatol. 139 (5): 897–901. doi:10.1046/j.1365-2133.1998.02522.x. PMID 9892963.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Secosteroids
- Drug