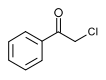
Phenacyl chloride
| |
| Ball-and-stick model | |
| Names | |
|---|---|
| IUPAC name
2-chloro-1-phenylethanone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| |
| |
| Properties | |
| C8H7ClO | |
| Molar mass | 154.59 g·mol−1 |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Phenacyl chloride |
|
Articles |
|---|
|
Most recent articles on Phenacyl chloride Most cited articles on Phenacyl chloride |
|
Media |
|
Powerpoint slides on Phenacyl chloride |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Phenacyl chloride |
|
Clinical Trials |
|
Ongoing Trials on Phenacyl chloride at Clinical Trials.gov Trial results on Phenacyl chloride Clinical Trials on Phenacyl chloride at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Phenacyl chloride NICE Guidance on Phenacyl chloride
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Phenacyl chloride Discussion groups on Phenacyl chloride Patient Handouts on Phenacyl chloride Directions to Hospitals Treating Phenacyl chloride Risk calculators and risk factors for Phenacyl chloride
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Phenacyl chloride |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Phenacyl chloride is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN.
Preparation
Phenacyl chloride is readily available commercially. It may be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst:[1]
Riot control agent
It was investigated, but not used, during the First and Second World Wars.
Because of its significantly greater toxicity,[2] it has largely been supplanted by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “Mace” or tear gas, its use is falling as pepper spray both works and disperses more quickly than CN.
The term "Mace" came into being because it was the brand-name invented by one of the first American manufacturers of CN aerosol sprays. Subsequently, Mace became synonymous with tear-gas sprays in the same way that Kleenex has become strongly associated with tissue papers (a phenomenon known as a genericized trademark).[citation needed]
Like CS gas, this compound irritates the mucous membranes (oral, nasal, conjunctival and tracheobronchial). Sometimes it can give rise to more generalized reactions such as syncope, temporary loss of balance and orientation.[2] More rarely, cutaneous irritating outbreaks have been observed and allergic contact permanent dermatitis.[3]
At high concentrations CN has caused corneal epithelial damage and chemosis. It has also accounted for at least five deaths, which have resulted from pulmonary injury and/or asphyxia.[4]
References
- ↑ Template:OrgSynth
- ↑ 2.0 2.1 Ballantyne, B.; Swanston, D. W. (1978). "The comparative acute mammalian toxicity of 1-chloroacetophenone (CN) and 2-chlorobenzylidene malononitrile (CS)". Archives of Toxicology. 40 (2): 75–95. doi:10.1007/BF01891962. PMID 350195.
- ↑ Template:Cite doi
- ↑ Blain, P. G. (2003). "Tear Gases and Irritant Incapacitants: 1-Chloroacetophenone, 2-Chlorobenzylidene Malononitrile and Dibenz[b,f]-1,4-Oxazepine". Toxicological Reviews. 22 (2): 103–110. PMID 15071820.
ar:غاز سي إن de:Ω-Chloracetophenon fa:کلرید فناسیل it:Gas CN kk:Хлорацетофенон lt:Chloracetofenonas nl:Chlooracetofenon sl:Kloracetofenon sv:Fenacylklorid th:แก๊สซีเอ็น
- Pages with script errors
- Pages with broken file links
- Articles without KEGG source
- Articles without UNII source
- Articles with changed EBI identifier
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Chembox
- Chembox having DSD data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- All articles with unsourced statements
- Articles with unsourced statements from July 2012
- Articles with invalid date parameter in template
- Riot control agents
- Lachrymatory agents