Onchocerciasis other diagnostic studies
|
Onchocerciasis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Onchocerciasis other diagnostic studies On the Web |
|
American Roentgen Ray Society Images of Onchocerciasis other diagnostic studies |
|
Risk calculators and risk factors for Onchocerciasis other diagnostic studies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Kalsang Dolma, M.B.B.S.[2]
Overview
The gold standard test for the diagnosis of onchocerciasis remains the skin snip biopsy. The biopsy is performed using a sclerocorneal biopsy punch or by elevating a small cone of skin (3 mm in diameter) with a needle and shaving it off with a scalpel. There are antibody tests that can assist in the diagnosis of onchocerciasis, though many are not available outside the research setting
Other Diagnostic Studies
Skin Snip Biopsy
The gold standard test for the diagnosis of onchocerciasis remains the skin snip biopsy. The biopsy is performed using a sclerocorneal biopsy punch or by elevating a small cone of skin (3 mm in diameter) with a needle and shaving it off with a scalpel. This will result in the removal of around 2 mg of tissue. The tissue is then incubated in normal saline at room temperature for 24 hours to allow the microfilariae to emerge. The microfilariae can then be identified microscopically. The sites for the skin snip are usually over the iliac crest, the scapula, and the lower extremities. Six snips provide the most diagnostic sensitivity. Although the skin snip is highly specific, its sensitivity can be limited in the pre-patent stage of infection, which can last 1 to 1.5 years, and in low intensity infections. Performing polymerase chain reaction (PCR) of the skin snip can increase the sensitivity in these two situations, though this is not commercially available. If a patient has skin nodules caused by onchocercal infection, nodulectomy allows for the identification of macrofilariae in the tissue. Slit lamp eye exam can be used to visualize microfilariae in individuals with eye disease.
Shown below is an image demonstrating a skin snip biopsy by elevating a piece of skin with a needle and shaving it off with a scalpel.
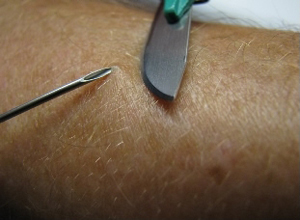
Shown below is an image demonstrating a skin snip biopsy using a sclerocorneal biopsy punch.
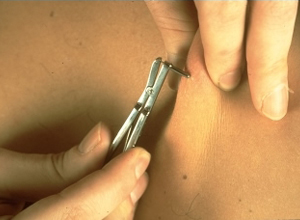
Antibody Tests
There are antibody tests that can assist in the diagnosis of onchocerciasis, though many are not available outside the research setting. There is a general screen for any filarial infection (including Wuchereria, Brugia, Loa, and Mansonella infections) that is available in some specialty diagnostic labs. Because the test is highly sensitive, it is useful in determining if an individual has had filarial infection, but it is not specific enough to identify which filarial infection. As with any antibody test, the results indicate only that the patient has been exposed to the disease, but it cannot determine if the patient has an active infection. This distinction is less important in symptomatic travelers, but it limits the usefulness of the test in persons from endemic areas. One advantage of the test is that it can pick up evidence of infection in the pre-patent stage of infection. There are several Onchocerca-specific serologic tests in existence, such as the OV-16 antigen antibody test and the OV luciferase immunoprecipitation system (LIPS) assay, but these are currently only available in the research setting and are not approved for diagnosis in the United States. In general the diagnosis of O. volvulus infection should be made with skin snip. However, when skin snips are negative and clinical suspicion of infection is high, the general antibody test could be used in an attempt to exclude infection. If the general antibody test were positive, then it might be necessary to consider performing additional skin snips and/or seeking additional diagnostic information by enlisting the assistance of researchers who perform antibody tests.