Nitrosamine
|
WikiDoc Resources for Nitrosamine |
|
Articles |
|---|
|
Most recent articles on Nitrosamine Most cited articles on Nitrosamine |
|
Media |
|
Powerpoint slides on Nitrosamine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Nitrosamine at Clinical Trials.gov Clinical Trials on Nitrosamine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Nitrosamine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Nitrosamine Discussion groups on Nitrosamine Patient Handouts on Nitrosamine Directions to Hospitals Treating Nitrosamine Risk calculators and risk factors for Nitrosamine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Nitrosamine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
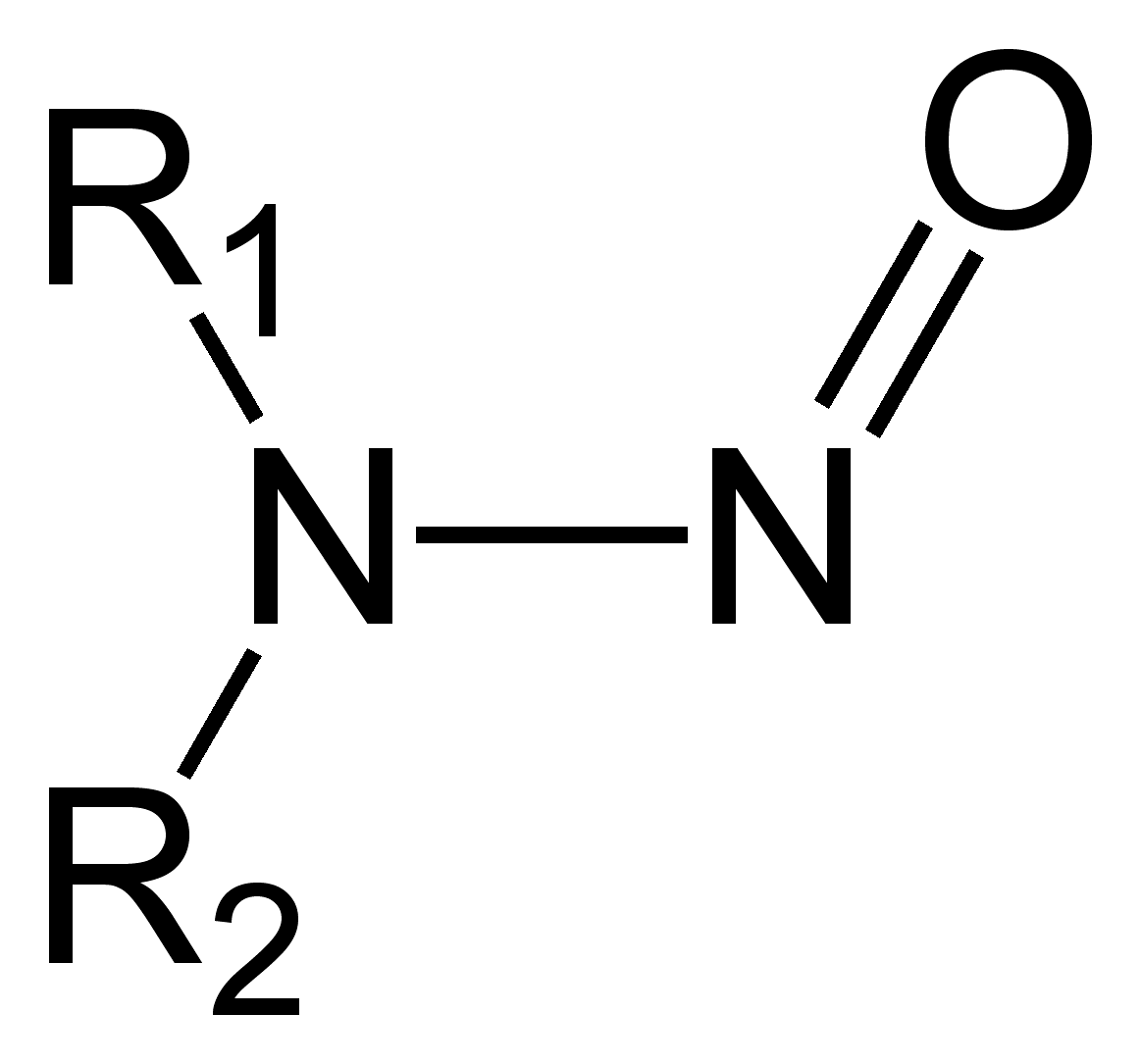
Nitrosamines are chemical compounds of the chemical structure R1N(-R2)-N=O, some of which are carcinogenic.
Occurrence in food
Nitrosamines are produced from nitrites and secondary amines, which often occur in the form of proteins. Their formation can occur only under certain conditions, including strongly acidic conditions such as that of the human stomach. High temperatures, as in frying, can also enhance the formation of nitrosamines.
The nitrite forms nitrous acid (HNO2), which splits into the nitrosonium cation, N=O+, and the hydroxide anion, OH−. The nitrosonium cation then reacts with an amine to produce nitrosamine.
Nitrosamines are found in many foodstuffs especially beer, fish, fish byproducts, and in meat and cheese products preserved with nitrite pickling salt. The US government established limits on the amount of nitrites used in meat products to decrease cancer risk in the population. There are also rules about adding ascorbic acid or related compounds to meat, because they inhibit nitrosamine formation.
Occurrence in other consumer products
Nitrosamines can be found in tobacco smoke and latex products. A test of party balloons and condoms indicated that many of them release small amounts of nitrosamines.[1] However, nitrosamines from condoms are not expected to be of toxicological significance.[1]
Cancer
Nitrosamines can cause cancers in a wide variety of animal species, a feature which suggests that they may also be carcinogenic in humans. Epidemiological data suggests that nitrosamines in preserved food cause stomach cancer.[2]
Uses
- Rubber products
- Pesticides
- Certain cosmetics
Examples of nitrosamines
Tobacco-specific nitrosamines
- N-Nitrosonornicotine (NNN)
- NNK
- NNAL
- NAT
- NAB
- iso-NNAL
- iso-NNAC
Other nitrosamines
See also
- Nitroamine (without the 's'), compounds of the formula R2N-NO2.
- Nitroso, compounds of the formula R-NO
External links
- Oregon State University, Linus Pauling Institute article on Nitrosamines and cancer, including info on history of meat laws
- Risk factors in Pancreatic Cancer
References
- ↑ Proksch E. Toxicological evaluation of nitrosamines in condoms. Int J Hyg Environ Health. 2001 Nov;204(2-3):103-10. PMID 11759152
- ↑ Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol. 2006 Jul 21;12(27):4296-303. PMID 16865769