Imidazoline
|
WikiDoc Resources for Imidazoline |
|
Articles |
|---|
|
Most recent articles on Imidazoline Most cited articles on Imidazoline |
|
Media |
|
Powerpoint slides on Imidazoline |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Imidazoline at Clinical Trials.gov Clinical Trials on Imidazoline at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Imidazoline
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Imidazoline Discussion groups on Imidazoline Patient Handouts on Imidazoline Directions to Hospitals Treating Imidazoline Risk calculators and risk factors for Imidazoline
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Imidazoline |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]}
Overview
Imidazoline is a nitrogen-containing heterocycle derived from imidazole. The ring contains an imine bond, and the carbons at the 4 and 5 positions are singly bonded, rather than doubly bonded for the case of imidazole. Imidazolines are structurally related to guanidines and amidines.
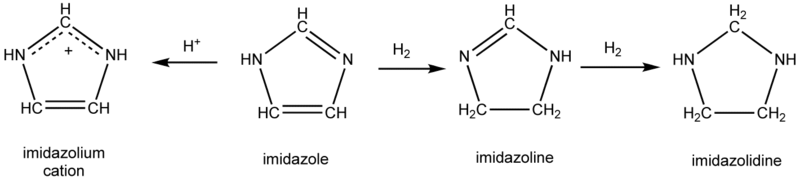
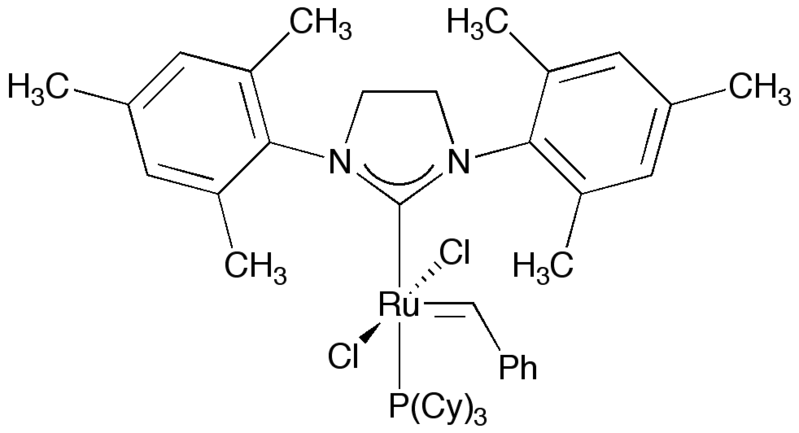
Like imidazole, imidazoline-based compounds have been used as N-heterocyclic carbene ligands on various transition metals. It is found in the commercially available second generation Grubbs' catalyst.
Biological role
Many imidazolines are biologically active.[1] Most bio-active derivatives bear a substituent (aryl or alkyl group) on the carbon between the nitrogen centers. Some brand names include oxymetazoline, xylometazoline, tetrahydrozoline, and naphazoline.
References
- ↑ N. MacInnes and S. Duty (2004). "Locomotor effects of imidazoline I2-site-specific ligands and monoamine oxidase inhibitors in rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway". Br J Pharmacol. 143 (8): 952–959. doi:10.1038/sj.bjp.0706019.