Pyridostigmine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pyridostigmine is a cholinesterase inhibitor that is FDA approved for the treatment of myasthenia gravis. Common adverse reactions include diaphoresis, diarrhea, excessive salivation, increased peristalsis, nausea and vomiting, stomach cramps, muscle fasciculation, asthenia, miosis and excessive bronchial secretion.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Myasthenia Gravis
Pyridostigmine bromide is available in tablets, each containing 60 mg pyridostigmine bromide.
- Dosage: The size and frequency of the dosage must be adjusted to the needs of the individual patient. The average dose is ten 60-mg tablets daily, spaced to provide maximum relief when maximum strength is needed. In severe cases as many as 25 tablets a day may be required, while in mild cases one to six tablets a day may suffice.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pyridostigmine (patient information) in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Organophosphate Poisoning
- Dosage: 30 mg q8h[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Myasthenia Gravis
Pyridostigmine bromide is available in tablets, each containing 60 mg pyridostigmine bromide.
- Dosage: The size and frequency of the dosage must be adjusted to the needs of the individual patient. The average dose is ten 60-mg tablets daily, spaced to provide maximum relief when maximum strength is needed. In severe cases as many as 25 tablets a day may be required, while in mild cases one to six tablets a day may suffice.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pyridostigmine (patient information) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pyridostigmine (patient information) in pediatric patients.
Contraindications
- Pyridostigmine bromide is contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma. Care should be observed in the use of atropine for counteracting side effects, as discussed below.
Warnings
- Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of pyridostigmine bromide may result in cholinergic crisis, a state characterized by increasing muscle weakness which, through involvement of the muscles of respiration, may lead to death. Myasthenic crisis due to an increase in the severity of the disease is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. Such differentiation is extremely important, since increases in doses of pyridostigmine bromide or other drugs of this class in the presence of cholinergic crisis or of a refractory or "insensitive" state could have grave consequences. *Osserman and Genkins indicate that the differential diagnosis of the two types of crisis may require the use of Tensilon (edrophonium chloride) as well as clinical judgment. The treatment of the two conditions obviously differs radically. Whereas the presence of myasthenic crisis suggests the need for more intensive anticholinesterase therapy, the diagnosis of cholinergic crisis, according to Osserman and Genkins, calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended.
- Atropine may also be used to abolish or obtund gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis. For detailed information on the management of patients with myasthenia gravis, the physician is referred to one of the excellent reviews such as those by Osserman and Genkins, Grob or Schwab.
Adverse Reactions
Clinical Trials Experience
- The side effects of pyridostigmine bromide are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis and diaphoresis. Nicotinic side effects are comprised chiefly of muscle cramps, fasciculation and weakness. Muscarinic side effects can usually be counteracted by atropine, but for reasons shown in the preceding section the expedient is not without danger. As with any compound containing the bromide radical, a skin rash may be seen in an occasional patient. Such reactions usually subside promptly upon discontinuance of the medication.
Postmarketing Experience
There is limited information regarding Pyridostigmine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Pyridostigmine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- The safety of pyridostigmine bromide during pregnancy or lactation in humans has not been established. Therefore, use of pyridostigmine bromide in women who may become pregnant requires weighing the drug's potential benefits against its possible hazards to mother and child.
Pregnancy Category (AUS): C
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pyridostigmine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pyridostigmine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Pyridostigmine in women who are nursing.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
There is no FDA guidance on the use of Pyridostigmine in geriatric settings.
Gender
There is no FDA guidance on the use of Pyridostigmine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pyridostigmine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pyridostigmine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pyridostigmine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pyridostigmine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pyridostigmine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Pyridostigmine bromide is available in tablets, each containing 60 mg pyridostigmine bromide.
Monitoring
There is limited information regarding Pyridostigmine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pyridostigmine and IV administrations.
Overdosage
There is limited information regarding Pyridostigmine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Pyridostigmine
| |
| Systematic (IUPAC) name | |
| 3-[(dimethylcarbamoyl)oxy]-1-methylpyridinium | |
| Identifiers | |
| CAS number | |
| ATC code | N07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
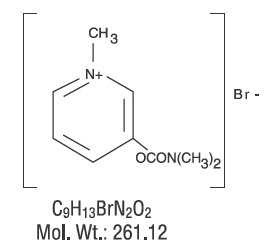
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 181.212 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 7.6 +/- 2.4% |
| Metabolism | ? |
| Half life | 1.78 +/- 0.24hrs |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral, intravenous |
Mechanism of Action
Pyridostigmine bromide inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction.
Structure
- Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:

Pharmacodynamics
- Pyridostigmine is an analog of neostigmine, but differs from it in certain clinically significant respects; for example, pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects.
Pharmacokinetics
There is limited information regarding Pyridostigmine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Pyridostigmine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Pyridostigmine Clinical Studies in the drug label.
How Supplied
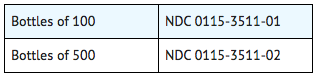
Storage
There is limited information regarding Pyridostigmine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pyridostigmine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
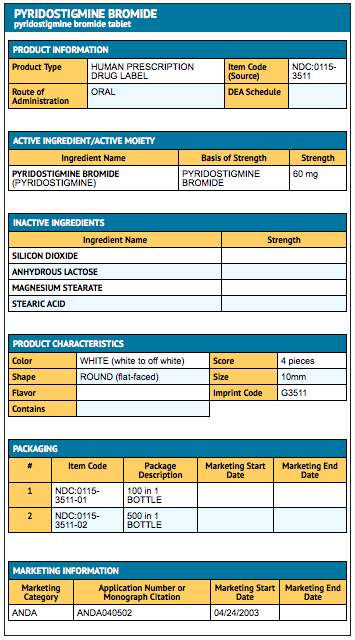
{{#ask: Label Page::Pyridostigmine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pyridostigmine Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Pyridostigmine (patient information) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Pyridostigmine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Keeler JR, Hurst CG, Dunn MA (1991). "Pyridostigmine used as a nerve agent pretreatment under wartime conditions". JAMA. 266 (5): 693–5. PMID 2072481.
{{#subobject:
|Label Page=Pyridostigmine |Label Name=Pyridostigmine Package.png
}}