Maralixibat
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Maralixibat is an ileal bile acid transporter inhibitor that is FDA approved for the treatment of cholestatic pruritus associated with Alagille syndrome. Common adverse reactions include liver test abnormalities, abdominal pain, fat-soluble vitamin deficiency, diarrhea, bone fractures, and gastrointestinal bleeding.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- 380 mcg/kg once daily, taken 30 minutes before the first meal of the day orally is recommended dosage in patients.
- 190 mcg/kg once daily is the recommended starting dosage for one week upon starting treatment of Maralixibat. After one week, increase dosage to recommended dosage of 380 mcg/kg.
- 3 mL per day is the maximum dosage in patients weighing above 70kg.
Table 1 shows Dosage of Maralixibat based on a Patient’s Weight.

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Maralixibat in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Maralixibat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Maralixibat FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Maralixibat in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Maralixibat in pediatric patients.
Contraindications
There are no contraindications associated with Maralixibat.
Warnings
Liver Test Abnormalities
- Trial studies show patients experiencing abnormal liver tests at the baseline.
- Comparing baseline liver test data in patients, liver tests worsened in some patients when taking Maralixibat.
- Patients also showed increases in AST, ALT, or T/DB.
- Increase in ALT lead to patients having dose interruptions (n=2), permanent discontinuation (n=2), or dose modifications (n=1) as shown from clinical studies.
- Increased TB above the baseline found in clinical trials can cause a patient to discontinue treatment with Maralixibat.
- Monitor patients liver function through liver tests when taking Maralixibat.
- Liver-related adverse reactions and elevations in liver tests should be cautioned to patients taking Maralixibat.
- Advise patients to discontinue Maralixibat if signs of portal hypertension persist in patients.
Gastrointestinal Adverse Reactions
- Abdominal pain, vomiting, and diarrhea was reported in patients who take Maralixibat.
- 3% of patients in clinical studies who experienced vomiting required hospitalization.
- Reduce or interrupt dosages of Maralixibat in patients experiencing abdominal pain, vomiting, and diarrhea.
- Monitor patients hydration levels if they display vomiting and diarrhea when taking Maralixibat.
- Reduce the dosage from 380 mcg/kg back to 190 mcg/kg/day and slowly increase after abdominal pain, vomiting, or diarrhea in patients has been resolved.
- Permanently discontinue use of Maralixibat if abdominal pain, vomiting, or diarrhea continue to be a persist in patients after resolution.
Fat-Soluble Vitamin (FSV) Deficiency
- Fat-soluble vitamins (vitamin A, D, E, and K) absorption may be affected by use of Maralixibat.
- Fat-soluble vitamins deficiency can occur in ALGS patients.
- Trial 1 clinical studies show 10% of patients reporting fat-soluble vitamins deficiency
- Monitor patients fat-soluble vitamins levels and supplement fat-soluble vitamins when deficiency occurs when taking Maralixibat.
- Permanently discontinue use of Maralixibat if fat-soluble vitamins levels do not improve after fat-soluble vitamins supplementation.
Adverse Reactions
Clinical Trials Experience
Clinical Trial Experience
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Alagille syndrome clinical development program
- This program was comprised of 5 clinical studies that looked into the adverse reactions of 86 patients being treated by Maralixibat. Patients received up to 760 mcg/kg per day of Maralixibat with the program that had a median duration of 32.3 months. 4-week placebo control period occurred in Trial 1 studies after 18 weeks of Maralixibat treatment. 13 weeks of placebo-controlled treatment occurred two long-term, open-label extension studies which specifically looked into patients receiving less than 380 mcg/kg/day dosages of Maralixibat. Dosage reductions and interruptions occurred 6% of patients that displayed vomiting, diarrhea, or abdominal pain in the studies conducted.
Table 2 shows Adverse Reactions (≥5%) caused by Maralixibat in ALGS patients.

Liver Test Abnormalities
- Pooled analysis of ALGS patients showed ALT increases with treatment of Maralixibat.
- ALT increases due to Maralixibat led to discontinuation (8.1%), and decreased dosage or dosage interruptions (3.5%) in patients.
- Cases of elevations was resolved through dosage modifications, dosage interruptions or no changes in dosage of Maralixibat
- 24% of patients showed increases to more than three times the baseline in ALT when using Maralixibat.
- 2% of patients showed increases to more than five times the baseline in ALT when using Maralixibat.
- 14% of patients showed AST increases to more than three times the baseline when using Maralixibat.
- 4.6% of patients showed bilirubin increases above baseline.
Postmarketing Experience
There is limited information about "Postmarketing Experiance" in the drug label.
Drug Interactions
Effects of Other Drugs on Maralixibat
Bile acid binding resins
- In the gut, Maralixibat may bind to bile acid binding resins.
- 4 hours before or 4 hours use of Maralixibat may have bile acid binding resins in the gut.
Effects of LIVMARLI on Other Drugs
OATP2B1 substrates
- In vitro studies, Maralixibat can be an OATP2B1 inhibitor.
- OATP2B1 inhibition may cause a decrease in the oral absorption of OATP2B1 substrates.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Clinical studies shows the systematic absorption of Maralixibat in the fetus is low for pregnant women taking the recommended dosage of Maralixibat orally. Fat-soluble vitamins absorption may be inhibited by use of Maralixibat. Studies done on pregnant rats, who received 1000 mg/kg/day of Maralixibat orally, and pregnant rabbits, who received 1000 mg/kg/day of Maralixibat orally, showed no effects were done to embryo development when using Maralixibat during a period of organogenesis. In female rats studies, Maralixibat had no effect on postnatal development when they received 750 mg/kg/day during organogenesis through lactation.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Maralixibat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Maralixibat during labor and delivery.
Nursing Mothers
At recommended dosages, use of Maralixibat should not be exposed to the fetus during lactation. No current data has been done on the effects of Maralixibat on the breastfed infant and the effects on milk production in women when treated with Maralixibat. Monitor patients during lactation for fat-soluble vitamins deficiency.
Pediatric Use
Safety and effectiveness of pediatric patients with cholestatic pruritus using Maralixibat has been established in a study. This study included patients ranging from 1 year of age to 15 years of age. There was 18 weeks of open-label treatment that was followed up by a 4 week period of placebo-controlled randomized withdrawal and an immediate open-label treatment period that lasted 26 weeks. 4 studies have provided safety information of patients up to 21 years of age using Maralixibat.
Geriatic Use
The safety and effectiveness of the use of Maralixibat in ALGS patients who are 65 years and older in age for the treatment of pruritus has not been established.
Gender
There is no FDA guidance on the use of Maralixibat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Maralixibat with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Maralixibat in patients with renal impairment.
Hepatic Impairment
Impaired hepatic function at baseline has been reported in ALGS patients treated with Maralixibat. Clinical studies on safety and effectiveness in both patients who have decompensated cirrhosis and portal hypertension have not been established.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Maralixibat in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance on the use of Maralixibat with respect to immunocompromised populations.
Administration and Monitoring
Administration
- Take recommended dosage as prescribed by the doctor 30 minutes before the first meal of the day.
- Patients should use calibrated measuring device from the pharmacy to measure Maralixibat dosage.
- If patients are taking a bile acid binding resin, do not take Maralixibat until at least 4 hours before or 4 hours after of the taking of bile acid binding resin.
- If a dosage is missed less than 12 hours of scheduled time, take dosage as soon as possible.
- If a dosage is missed by more than 12 hours, then skip dosage and take next dosage at scheduled time.
Monitoring
- Monitor patients liver tests throughout treatment with MAralixibat and compare the results to the baseline.
- Monitor patients TB, AST, and International Normalized Ratio levels when taking Maralixibat.
- Any liver test abnormalities should lead to interruption in Maralixibat dosage.
- If liver test abnormalities become stabilized, start patients with 190 mcg/kg of Maralixibat and work dosage back up to 380 mcg/kg if tolerated by patients.
- Hepatic decompensation events in patients should lead to permanent discontinuation of Maralixibat treatment.
IV Compatibility
There is limited information regarding the compatibility of Maralixibat and IV administrations.
Overdosage
- 500 mg, approximately 18-fold higher than the recommended dose, of Maralixibat in a single dose regimen has showed no indications of meaningful increase in adverse reactions in patients when compared to reactions occurring in lower dosage amounts.
- Monitor patients signs and symptoms when overdosed on Maralixibat.
- Propylene glycol (364.5 mg/mL) is found in Maralixibat.
- 50 mg/kg/day and 500 mg/kg/day of propylene glycol are considered safe in the body.
- CNS, hyperosmolality, cardiovascular, and/or respiratory effects may be present in patients who overdose on propylene glycol found in Maralixibat.
Pharmacology
Mechanism of Action
- Maralixibat is a reversible inhibitor of the ileal bile acid transporter.
- In the terminal ileum, the reabsorption of bile acids is decreased in the presence of Maralixibat.
- Inhibition of the ileal bile acid transporter inhibitor may occur in the presence of Maralixibat which can decrease bile salts re-uptake.
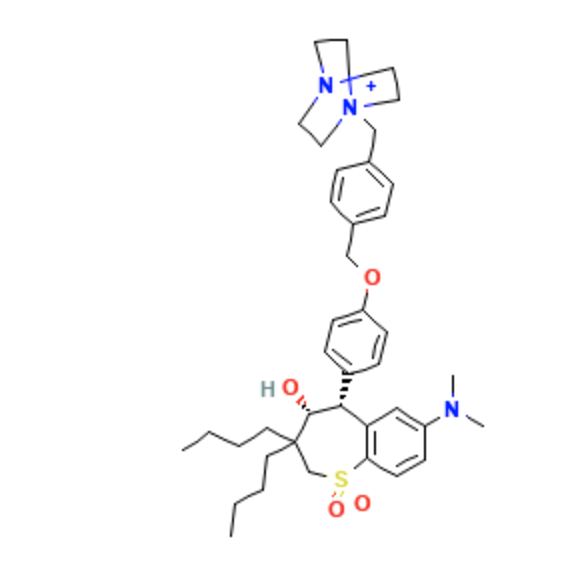
Structure
- Maralixibat is an ileal bile acid transporter inhibitor for oral administration.
- It has an empirical formula of C40H56ClN3O4S and a molecular weight of 710.42 g/mol.
- The chemical name is 1-[4-({4-[(4R,5R)-3,3-Dibutyl-7-(dimethylamino)-4-hydroxy-1,1-dioxido-2,3,4,5-tetrahydro-1-benzothiepin-5-yl]phenoxy}methyl)benzyl]-4-aza-1-azoniabicyclo[2.2.2]octane
Pharmacodynamics
- Trial 1 studies looked into serum bile acids levels in pediatric patients taking Maralixibat.
- Pediatric patients with ALGS started an initial 5-week dose-escalation period which was followed by open-label treatment for 13 weeks of 380 mcg/kg of a single daily dosage of Maralixibat.
- Patients in the study had serum bile acids levels that ranged from 20 to 749 µmol/L for the baseline.
- The mean of serum bile acid level was 283 (210.6) µmol/L of pediatric patients at baseline.
- As early as Week 12, patients reported decreases in serum bile acid levels when compared to the baseline.
Pharmacokinetics
Pharmacokinetic Parameters
- Pharmacokinetics of Maralixibat is not calculated reliably due to low systemic absorption of Maralixibat.
- Maralixibat was below the limit of quantification in pediatric ALGS patients.
- Trial 1 studies show 5.93 ng/mL is the highest concentrations of Maralixibat in pediatric patients receiving 380 mcg/kg once daily of Maralixibat.
- Plasma concentrations are below the limit of quantification with doses less than 20 mg of Maralixibat in healthy adults.
- 0.75 is the median Tmax after patients received a single 30 mg under fasted condition dosage of Maralixibat.
- 1.65 (1.10) ng/m is the mean (SD) Cmax after patients received a single 30 mg under fasted condition dosage of Maralixibat.
- 3.43 (2.13) ng∙h/mL is the mean (SD) AUClast after patients received a single 30 mg under fasted condition dosage of Maralixibat.
Absorption
- Plasma concentrations of Maralixibat are below the limit of quantification.
- Absorption of Maralixibat is minimally absorbed.
- AUClast increased 4.6 fold when looking at patients receiving 30, 45, and 100 mg of Maralixibat once daily under fasted condition.
- Cmax increased 2.4 fold when looking at patients receiving 30, 45, and 100 mg of Maralixibat once daily under fasted condition.
- Healthy adults receiving up to 100 mg of Maralixibat showed no accumulation.
Effect of Food
- Patients who eat a high-fat meal showed a decrease in both extent and rate of absorption with concomitant use of Maralixibat.
- Changes of systemic exposures by the effect of food in patients taking Maralixibat is not clinically significant.
Distribution
- In vitro, Maralixibat has a plasma protein binding percentage of 91%.
Elimination
- The mean half-life is 1.6 hours in patients receiving 30 mg Maralixibat once daily.
Metabolism
- Plasma had no signs of Maralixibat metabolites.
- (14C)Maralixibat orally administered showed <3% of three minor metabolites in total fecal radioactivity.
Excretion
- Major route of elimination is fecal excretion.
- In feces, after oral administration of 5 mg 14C-Maralixibat, 73% of Maralixibat was found in which 94% was found unchanged.
- In urine, after oral administration of 5 mg 14C-Maralixibat, 0.066% of Maralixibat was found.
Specific Populations
Patients with Renal Impairment
- The pharmacokinetics of patients who have impaired renal function receiving Maralixibat has not been studied.
Drug Interaction Studies
Effect of Other Drugs on Maralixibat
- Maralixibat for certain drug transporters such as BCRP, OATP1B1, MDR1, OATP2B2, or OATP1B3 is not a substrate.
- Disposition of Maralixibat is predicted to not be affected by concomitant drug transporters.
Effect of Maralixibat on Other Drugs
- In vitro, CYP isoforms 2B6, 3A4, or 1A2 are not induced by Maralixibat. CYP isoforms 2C9, 2C19, 2B6, 1A2, 2D6 or 2C8 are not inhibited by Maralixibat. CYP3A4 is inhibited by Maralixibat, but it is unlikely that the pharmacokinetics of CYP3A4 will have relevant effects. Transporters such as OAT1, OAT3, OATP1B1, OCT1, OCT2, OCT3, OCTN1, BCRP, MRP2, MATE1, MDR1, MATE2-K, or OCTN2 are not inhibited by Maralixibat.
- In vitro, Drug transporter OATP2B1 is inhibited by Maralixibat which may reduce absorption for OATP2B1-mediated uptake drugs.
- Pharmacokinetics of statins and metabolites have no relevant effect when simvastatin or lovastatin is co-administered with 4.75 mg of Maralixibat.
- Pharmacokinetics of atorvastatin is not effected with co-administration of 4.75 mg Maralixibat.
- Clinical study is needed to look into the pharmacokinetics of OATP2B1 substrates at higher doses when co-administered with 4.75 mg of Maralixibat to see if Maralixibat has an effect.
Nonclinical Toxicology
Carcinogenesis
- TgRasH2 mice did not show any drug-related tumors when taking Maralixibat for 26 weeks.
Mutagenesis
- In vivo and vitro assays, Maralixibat was negative for mutagenesis.
Impairment of Fertility
- Female rats who received up to 2000 mg/kg/day of Maralixbat and male rats who received up to 750 mg/kg/day of Maralixibat showed No effects on their fertility when treated with Maralixibat.
Clinical Studies
Trial 1 Study
- The study conducted included a 4-week randomized, double-blind, placebo-controlled drug-withdrawal period; an 18-week open-label treatment period; and a subsequent 26-week open-label treatment period; and a long-term open-label extension period. The study was made up of 31 ALGS, pediatric patients that had pruritus, the JAGGED Mutation, and cholestasis. The patient population included 66% males with a median of 5 years in age. Patients in the study had a baseline AST level of 158 (68) U/L, baseline ALT of 179 (112) U/L, baseline GGT of 498 (399) U/L, baseline serum bile acid levels of 280 (213) µmol/L, and a baseline TB level of 5.6 (5.4) mg/dL. 90.3% of the patients in the study with pruritus received one medication for treatment of the pruritus. Patients started with a 5-week dose-escalation period that was followed by 13 weeks of Maralixibat at 380 mcg/kg once daily. Two patients in the study discontinued the use of Maralixibat first 18 weeks of open-label treatment dropping the patient population to 29 patients. The 29 patients left were then put in a 4-week drug withdrawal period with the patients either receiving a placebo or continued with Maralixibat. Afterward, all patients received 380 mcg/kg once daily of Maralixibat for 26 more weeks. A 5-point ordinal response scale was used on the pediatric patients that show symptoms of pruritus. The mean baseline score of pruritus is 3.1 in patients pre-treatment with 1.4 as the mean score at Week 18. There was a reduction in pruritus for patients who received Maralixibat for 22 weeks. The baseline score was similar to the score in patients who received the placebo after 18 weeks of Maralixibat. Similar pruritus scores were seen in all patients during the open-label treatment phase.
Table 3 shows Patient Pruritus Scores during the Placebo-Controlled Period.

How Supplied
- 30 mL of Maralixibat with 9.5 mg of Maralixibat for each mL in the plastic bottle.
- Marlixibat is a clear, colorless to yellow liquid that is orally administered in patients.
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
- Advise patients to throw out any Maralixibat remaining after 45 days of first opening the bottle.
- Advise patients to put cap back on Maralixibat bottle after daily usage.
Images
Drug Images
{{#ask: Page Name::Maralixibat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Maralixibat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Risks
- Advise patients about reported symptoms when taking Maralixibat such as vomiting, diarrhea, dehydration, and abdominal pain.
- Advise patients to seek medical attention if symptoms worsen when taking Maralixibat.
- Patients have experienced liver test elevations when taking Maralixibat.
- Monitor patients liver test results when taking Maralixibat to the baseline.
- Advise patients to seek medical attention if liver problems arise such as dark or brown urine and possibly pain on the right side of the abdomen.
- Absorption of fat-soluble vitamins may decrease with treatment of Maralixibat.
- Monitor patients fat-soluble vitamins levels from start to end of treatment of Maralixibat to make sure no fat-soluble vitamins deficiency is not occurring in the patient.
Administration
- Maralixibat should be taken 30 minutes before the first meal of the day orally.
- Measure dosage of Maralixibat with a calibrated measuring device that will be provided to patients by their pharmacist.
- Advise patients to throw out any Maralixibat remaining after 45 days of first opening the bottle.
- Patients taking a bile acid binding resin should wait on taking Maralixibat until at least 4 hours before or 4 hours after taking the bile acid binding resin.
Precautions with Alcohol
Alcohol-Maralixibat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Livmarli
Look-Alike Drug Names
There is limited information regarding Maralixibat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.