Ledipasvir / sofosbuvir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
See full prescribing information for complete Boxed Warning.
*Test all patients for evidence of current or prior hepatitis B virus (HBV) infection before initiating treatment with Ledipasvir/Sofosbuvir. HBV reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals and were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Monitor HCV/HBV coinfected patients for hepatitis flare or HBV reactivation during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated.
|
Overview
Ledipasvir / sofosbuvir is a fixed-dose combination of Ledipasvir, a hepatitis C virus (HCV) NS5A inhibitor, and Sofosbuvir, an HCV nucleotide analog NS5B polymerase inhibitor that is FDA approved for the treatment of chronic hepatitis C virus (HCV). There is a Black Box Warning for this drug as shown here. Common adverse reactions include fatigue, headache and asthenia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Adult Patient Indications:
- Ledipasvir/Sofosbuvir is indicated for the treatment of adult patients with chronic hepatitis C virus (HCV):
- genotype 1, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis.
- genotype 1 infection with decompensated cirrhosis, for use in combination with ribavirin.
- genotype 1 or 4 infection who are liver transplant recipients without cirrhosis or with compensated cirrhosis, for use in combination with ribavirin.
Recommended Dosage in Adults:
- The recommended dosage of Ledipasvir/Sofosbuvir is one tablet (90 mg Ledipasvir and 400 mg Sofosbuvir) taken orally once daily with or without food.
- Relapse rates are affected by baseline host and viral factors and differ between treatment durations for certain subgroups.
- Table 1 shows the recommended Ledipasvir/Sofosbuvir treatment regimen and duration based on patient population.
- For patients with HCV/HIV-1 coinfection, follow the dosage recommendations in Table 1. Refer to Drug Interactions (7) for dosage recommendations for concomitant HIV-1 antiviral drugs.
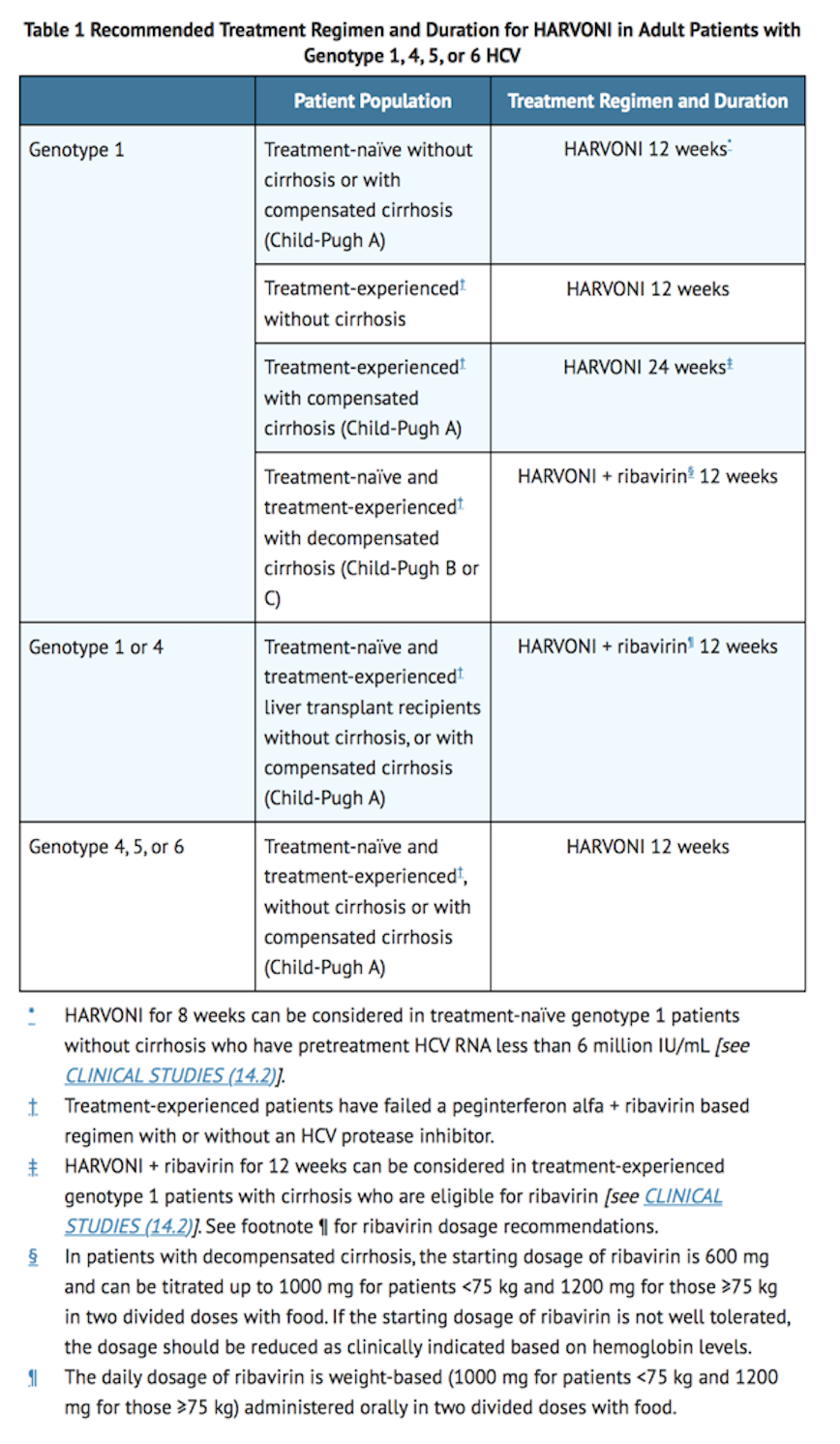
- For further information on ribavirin dosing and dosage modifications, refer to the ribavirin prescribing information.
Severe Renal Impairment and End Stage Renal Disease
- No dosage recommendation can be given for patients with severe renal impairment (estimated Glomerular Filtration Rate [eGFR] less than 30 mL/min/1.73 m2) or with end stage renal disease (ESRD) due to higher exposures (up to 20-fold) of the predominant Sofosbuvir metabolite.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Ledipasvir/Sofosbuvir Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Ledipasvir/Sofosbuvir Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Pediatric Patient Indications:
- Ledipasvir/Sofosbuvir is indicated for the treatment of pediatric patients 12 years of age and older or weighing at least 35 kg with HCV genotype 1, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis.
Recommended Dosage in Pediatric Patients 12 Years of Age and Older or Weighing at Least 35 kg:
- The recommended dosage of Ledipasvir/Sofosbuvir in pediatric patients 12 years of age and older or weighing at least 35 kg is one tablet (90 mg Ledipasvir and 400 mg Sofosbuvir) taken orally once daily with or without food for 12 weeks.
- Table 2 shows the recommended Ledipasvir/Sofosbuvir duration based on pediatric patient population.
- For patients with HCV/HIV-1 coinfection, follow the dosage recommendations in Table 2.

Severe Renal Impairment and End Stage Renal Disease
- No dosage recommendation can be given for patients with severe renal impairment (estimated Glomerular Filtration Rate [eGFR] less than 30 mL/min/1.73 m2) or with end stage renal disease (ESRD) due to higher exposures (up to 20-fold) of the predominant Sofosbuvir metabolite.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Ledipasvir/Sofosbuvir Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Ledipasvir/Sofosbuvir Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- If Ledipasvir/Sofosbuvir is administered with ribavirin, the contraindications to ribavirin also apply to this combination regimen. Refer to the ribavirin prescribing information for a list of contraindications for ribavirin.
Warnings
|
RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
See full prescribing information for complete Boxed Warning.
*Test all patients for evidence of current or prior hepatitis B virus (HBV) infection before initiating treatment with Ledipasvir/Sofosbuvir. HBV reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals and were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Monitor HCV/HBV coinfected patients for hepatitis flare or HBV reactivation during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated.
|
Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
- Hepatitis B virus (HBV) reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals, and who were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Cases have been reported in patients who are HBsAg positive and also in patients with serologic evidence of resolved HBV infection (i.e., HBsAg negative and anti-HBc positive). HBV reactivation has also been reported in patients receiving certain immunosuppressants or chemotherapeutic agents; the risk of HBV reactivation associated with treatment with HCV direct-acting antivirals may be increased in these patients.
- HBV reactivation is characterized as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level. In patients with resolved HBV infection, reappearance of HBsAg can occur. Reactivation of HBV replication may be accompanied by hepatitis, i.e., increases in aminotransferase levels and, in severe cases, increases in bilirubin levels, liver failure, and death can occur.
- Test all patients for evidence of current or prior HBV infection by measuring HBsAg and anti-HBc before initiating HCV treatment with Ledipasvir/Sofosbuvir. In patients with serologic evidence of HBV infection, monitor for clinical and laboratory signs of hepatitis flare or HBV reactivation during HCV treatment with Ledipasvir/Sofosbuvir and during post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated.
Serious Symptomatic Bradycardia When Coadministered with Amiodarone
- Postmarketing cases of symptomatic bradycardia, as well as fatal cardiac arrest and cases requiring pacemaker intervention, have been reported when amiodarone is coadministered with Ledipasvir/Sofosbuvir. Bradycardia has generally occurred within hours to days, but cases have been observed up to 2 weeks after initiating HCV treatment. Patients also taking beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease, may be at increased risk for symptomatic bradycardia with coadministration of amiodarone. Bradycardia generally resolved after discontinuation of HCV treatment. The mechanism for this effect is unknown.
- Coadministration of amiodarone with Ledipasvir/Sofosbuvir is not recommended. For patients taking amiodarone who have no other alternative, viable treatment options and who will be coadministered Ledipasvir/Sofosbuvir:
- Counsel patients about the risk of serious symptomatic bradycardia.
- Cardiac monitoring in an in-patient setting for the first 48 hours of coadministration is recommended, after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.
- Patients who are taking Ledipasvir/Sofosbuvir who need to start amiodarone therapy due to no other alternative, viable treatment options should undergo similar cardiac monitoring as outlined above.
- Due to amiodarone's long half-life, patients discontinuing amiodarone just prior to starting Ledipasvir/Sofosbuvir should also undergo similar cardiac monitoring as outlined above.
- Patients who develop signs or symptoms of bradycardia should seek medical evaluation immediately. Symptoms may include near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pains, confusion or memory problems.
Risk of Reduced Therapeutic Effect Due to Use with P-gp Inducers
- The concomitant use of Ledipasvir/Sofosbuvir and P-gp inducers (e.g., rifampin, St. John's wort) may significantly decrease Ledipasvir and Sofosbuvir plasma concentrations and may lead to a reduced therapeutic effect of Ledipasvir/Sofosbuvir. Therefore, the use of Ledipasvir/Sofosbuvir with P-gp inducers (e.g., rifampin or St. John's wort) is not recommended.
Risks Associated with Ribavirin Combination Treatment
- If Ledipasvir/Sofosbuvir is administered with ribavirin, the warnings and precautions for ribavirin, in particular the pregnancy avoidance warning, apply to this combination regimen. Refer to the ribavirin prescribing information for a full list of the warnings and precautions for ribavirin.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- If Ledipasvir/Sofosbuvir is administered with ribavirin to adults, refer to the prescribing information for ribavirin for a description of ribavirin-associated adverse reactions.
Clinical Trials in Adult Subjects
- The safety assessment of Ledipasvir/Sofosbuvir was based on pooled data from three randomized, open-label Phase 3 clinical trials (ION-3, ION-1, and ION-2) of subjects with genotype 1 HCV with compensated liver disease (with and without cirrhosis) including 215, 539, and 326 subjects who received Ledipasvir/Sofosbuvir once daily by mouth for 8, 12 and 24 weeks, respectively.
- The proportion of subjects who permanently discontinued treatment due to adverse events was 0%, less than 1%, and 1% for subjects receiving Ledipasvir/Sofosbuvir for 8, 12, and 24 weeks, respectively.
- The most common adverse reactions (at least 10%) were fatigue and headache in subjects treated with 8, 12, or 24 weeks of Ledipasvir/Sofosbuvir.
- Table 3 lists adverse reactions (adverse events assessed as causally related by the investigator, all grades) observed in at least 5% of subjects receiving 8, 12, or 24 weeks treatment with Ledipasvir/Sofosbuvir in clinical trials. The majority of adverse reactions presented in Table 3 occurred at severity of grade 1. The side-by-side tabulation is to simplify presentation; direct comparison across trials should not be made due to differing trial designs.

- The safety assessment of Ledipasvir/Sofosbuvir was also based on pooled data from three open-label trials (Study 1119, ION-4, and ELECTRON-2) in 118 subjects with chronic HCV genotype 4, 5, or 6 infection with compensated liver disease (with or without cirrhosis). The subjects received Ledipasvir/Sofosbuvir once daily by mouth for 12 weeks. The safety profile in subjects with chronic HCV genotype 4, 5, or 6 infection with compensated liver disease was similar to that observed in subjects with chronic HCV genotype 1 infection with compensated liver disease. The most common adverse reactions occurring in at least 10% of subjects were asthenia (18%), headache (14%), and fatigue (10%).
Adverse Reactions in Subjects with Cirrhosis
- The safety assessment of Ledipasvir/Sofosbuvir with or without ribavirin was based on a randomized, double-blind and placebo-controlled trial in treatment-experienced genotype 1 subjects with compensated cirrhosis and was compared to placebo in the SIRIUS trial. Subjects were randomized to receive 24 weeks of Ledipasvir/Sofosbuvir once daily by mouth without ribavirin or 12 weeks of placebo followed by 12 weeks of Ledipasvir/Sofosbuvir once daily by mouth + ribavirin. Table 4 presents the adverse reactions, as defined above, that occurred with at least 5% greater frequency in subjects treated with 24 weeks of Ledipasvir/Sofosbuvir or 12 weeks of Ledipasvir/Sofosbuvir + ribavirin, compared to those reported for 12 weeks of placebo. The majority of the adverse reactions presented in Table 4 were Grade 1 or 2 in severity.

Adverse Reactions in Subjects Coinfected with HIV-1
- The safety assessment of Ledipasvir/Sofosbuvir was based on an open-label clinical trial in 335 genotype 1 or 4 subjects with HCV/HIV-1 coinfection who were on stable antiretroviral therapy in Study ION-4. The safety profile in HCV/HIV-1 coinfected subjects was similar to that observed in HCV mono-infected subjects. The most common adverse reactions occurring in at least 10% of subjects were headache (20%) and fatigue (17%).
Adverse Reactions in Liver Transplant Recipients and/or Subjects with Decompensated Cirrhosis
- The safety assessment of Ledipasvir/Sofosbuvir with ribavirin (RBV) in liver transplant recipients and/or those who had decompensated liver disease was based on pooled data from two Phase 2 open-label clinical trials including 336 subjects who received Ledipasvir/Sofosbuvir plus RBV for 12 weeks. Subjects with Child-Pugh-Turcotte (CPT) scores greater than 12 were excluded from the trials.
- The adverse events observed were consistent with the expected clinical sequelae of liver transplantation and/or decompensated liver disease, or the known safety profile of Ledipasvir/Sofosbuvir and/or ribavirin.
- Decreases in hemoglobin to less than 10 g/dL and 8.5 g/dL during treatment were observed in 38% and 13% of subjects treated with Ledipasvir/Sofosbuvir plus RBV for 12 weeks, respectively. Ribavirin was permanently discontinued in 11% of subjects treated with Ledipasvir/Sofosbuvir plus RBV for 12 weeks.
Liver Transplant Recipients with Compensated Liver Disease:
- Among the 174 liver transplant recipients with compensated liver disease who received Ledipasvir/Sofosbuvir with RBV for 12 weeks, 2 (1%) subjects permanently discontinued Ledipasvir/Sofosbuvir due to an adverse event.
Subjects with Decompensated Liver Disease:
- Among the 162 subjects with decompensated liver disease (pre- or post-transplant) who received Ledipasvir/Sofosbuvir with RBV for 12 weeks, 7 (4%) subjects died, 4 (2%) subjects underwent liver transplantation, and 1 subject (<1%) underwent liver transplantation and died during treatment or within 30 days after discontinuation of treatment. Because these events occurred in patients with advanced liver disease who are at risk of progression of liver disease including liver failure and death, it is not possible to reliably assess the contribution of drug effect to outcomes. A total of 4 (2%) subjects permanently discontinued Ledipasvir/Sofosbuvir due to an adverse event.
- Less Common Adverse Reactions Reported in Clinical Trials (less than 5%): The following adverse reactions occurred in less than 5% of subjects receiving Ledipasvir/Sofosbuvir in any one trial. These events have been included because of their seriousness or assessment of potential causal relationship.
- Psychiatric disorders: depression (including in subjects with pre-existing history of psychiatric illness).
- Depression (particularly in subjects with pre-existing history of psychiatric illness) occurred in subjects receiving Sofosbuvir containing regimens. Suicidal ideation and suicide have occurred in less than 1% of subjects treated with Sofosbuvir in combination with ribavirin or pegylated interferon/ribavirin in other clinical trials.
Laboratory Abnormalities
- Bilirubin Elevations: Bilirubin elevations of greater than 1.5×ULN were observed in 3%, less than 1%, and 2% of subjects treated with Ledipasvir/Sofosbuvir for 8, 12, and 24 weeks, respectively. Bilirubin elevations of greater than 1.5×ULN were observed in 3%, 11%, and 3% of subjects with compensated cirrhosis treated with placebo, Ledipasvir/Sofosbuvir + ribavirin for 12 weeks and Ledipasvir/Sofosbuvir for 24 weeks, respectively, in the SIRIUS trial.
- Lipase Elevations: Transient, asymptomatic lipase elevations of greater than 3×ULN were observed in less than 1%, 2%, and 3% of subjects treated with Ledipasvir/Sofosbuvir for 8, 12, and 24 weeks, respectively. Transient, asymptomatic lipase elevations of greater than 3× ULN were observed in 1%, 3%, and 9% of subjects with compensated cirrhosis treated with placebo, Ledipasvir/Sofosbuvir + ribavirin for 12 weeks, and Ledipasvir/Sofosbuvir for 24 weeks, respectively, in the SIRIUS trial.
- Creatine Kinase: Creatine kinase was not assessed in Phase 3 trials ION-3, ION-1, or ION-2 of Ledipasvir/Sofosbuvir. Creatine kinase was assessed in the ION-4 trial. Isolated, asymptomatic creatine kinase elevations of greater than or equal to 10×ULN was observed in 1% of subjects treated with Ledipasvir/Sofosbuvir for 12 weeks in the ION-4 trial and has also been previously reported in subjects treated with Sofosbuvir in combination with ribavirin or peginterferon/ribavirin in other clinical trials.
Adverse Reactions in Pediatric Subjects 12 Years of Age and Older
- The safety assessment of Ledipasvir/Sofosbuvir in pediatric subjects 12 years of age and older is based on data from a Phase 2, open-label clinical trial (Study 1116) that enrolled 100 subjects without cirrhosis or with compensated cirrhosis who were treated with Ledipasvir/Sofosbuvir for 12 weeks. The adverse reactions observed were consistent with those observed in clinical studies of Ledipasvir/Sofosbuvir in adults. Limited safety data are available in pediatric subjects receiving Ledipasvir/Sofosbuvir for 24 weeks. No Grade 3 or 4 adverse reactions or discontinuation due to an adverse reaction was observed in those pediatric subjects receiving Ledipasvir/Sofosbuvir for 24 weeks.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of Ledipasvir/Sofosbuvir. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders
- Serious symptomatic bradycardia has been reported in patients taking amiodarone who initiate treatment with Ledipasvir/Sofosbuvir.
Skin and Subcutaneous Tissue Disorders
- Skin rashes, sometimes with blisters or angioedema-like swelling.
- Angioedema.
Drug Interactions
- Potential for Drug Interaction
- Established and Potentially Significant Drug Interactions
- Drugs without Clinically Significant Interactions with Ledipasvir/Sofosbuvir
Potential for Drug Interaction
- As Ledipasvir/Sofosbuvir contains Ledipasvir and Sofosbuvir, any interactions that have been identified with these agents individually may occur with Ledipasvir/Sofosbuvir.
- After oral administration of Ledipasvir/Sofosbuvir, Sofosbuvir is rapidly absorbed and subject to extensive first-pass hepatic extraction. In clinical pharmacology studies, both Sofosbuvir and the inactive metabolite GS-331007 were monitored for purposes of pharmacokinetic analyses.
- Ledipasvir is an inhibitor of the drug transporters P-gp and breast cancer resistance protein (BCRP) and may increase intestinal absorption of coadministered substrates for these transporters.
- Ledipasvir and Sofosbuvir are substrates of drug transporters P-gp and BCRP while GS-331007 is not. P-gp inducers (e.g., rifampin or St. John's wort) may decrease Ledipasvir and Sofosbuvir plasma concentrations, leading to reduced therapeutic effect of Ledipasvir/Sofosbuvir, and the use with P-gp inducers is not recommended with Ledipasvir/Sofosbuvir.
- Fluctuations in INR values may occur in patients receiving warfarin concomitant with HCV treatment, including treatment with Ledipasvir/Sofosbuvir. Frequent monitoring of INR values is recommended during treatment and post-treatment follow-up.
Established and Potentially Significant Drug Interactions
- Table 5 provides a listing of established or potentially clinically significant drug interactions. The drug interactions described are based on studies conducted with either Ledipasvir/Sofosbuvir, the components of Ledipasvir/Sofosbuvir (Ledipasvir and Sofosbuvir) as individual agents, or are predicted drug interactions that may occur with Ledipasvir/Sofosbuvir.

Drugs without Clinically Significant Interactions with Ledipasvir/Sofosbuvir
- Based on drug interaction studies conducted with the components of Ledipasvir/Sofosbuvir (Ledipasvir or Sofosbuvir) or Ledipasvir/Sofosbuvir, no clinically significant drug interactions have been either observed or are expected when Ledipasvir/Sofosbuvir is used with the following drugs: abacavir, atazanavir/ritonavir, cyclosporine, darunavir/ritonavir, dolutegravir, efavirenz, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, emtricitabine, lamivudine, methadone, oral contraceptives, pravastatin, raltegravir, rilpivirine, tacrolimus, or verapamil. See TABLE 5 for use of Ledipasvir/Sofosbuvir with certain HIV antiretroviral regimens.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- If Ledipasvir/Sofosbuvir is administered with ribavirin, the combination regimen is contraindicated in pregnant women and in men whose female partners are pregnant. Refer to the ribavirin prescribing information for more information on ribavirin-associated risks of use during pregnancy.
- No adequate human data are available to establish whether or not Ledipasvir/Sofosbuvir poses a risk to pregnancy outcomes. In animal reproduction studies, no evidence of adverse developmental outcomes was observed with the components of Ledipasvir/Sofosbuvir (Ledipasvir or Sofosbuvir) at exposures greater than those in humans at the recommended human dose (RHD). During organogenesis in the rat and rabbit, systemic exposures (AUC) to Ledipasvir were approximately 4 (rats) and 2 (rabbits) times the exposure in humans at the RHD, while exposures to the predominant circulating metabolite of Sofosbuvir (GS-331007) were ≥3 (rats) and 7 (rabbits) times the exposure in humans at the RHD. In rat pre/postnatal development studies, maternal systemic exposures (AUC) to Ledipasvir and GS-331007 were approximately 5 and 7 times, respectively, the exposure in humans at the RHD.
- The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data (Animal)
- Ledipasvir: Ledipasvir was administered orally to pregnant rats (up to 100 mg/kg/day) and rabbits (up to 180 mg/kg/day) on gestation days 6 to 18 and 7 to 20, respectively, and also to rats (oral doses up to 100 mg/kg/day) on gestation day 6 to lactation/post-partum day 20. No significant effects on embryo-fetal (rats and rabbits) or pre/postnatal (rats) development were observed at the highest doses tested. Systemic exposures (AUC) to Ledipasvir were ≥4 (rats) and 2 (rabbits) times the exposure in humans at the RHD.
- Sofosbuvir: Sofosbuvir was administered orally to pregnant rats (up to 500 mg/kg/day) and rabbits (up to 300 mg/kg/day) on gestation days 6 to 18 and 6 to 19, respectively, and also to rats (oral doses up to 500 mg/kg/day) on gestation day 6 to lactation/post-partum day 20. No significant effects on embryo-fetal (rats and rabbits) or pre/postnatal (rats) development were observed at the highest doses tested. Systemic exposures (AUC) to the predominant circulating metabolite of Sofosbuvir (GS-331007) were ≥3 (rats) and 7 (rabbits) times the exposure in humans at the RHD, with exposures increasing during gestation from approximately 3 to 6 (rats) and 7 to 17 (rabbits) times the exposure in humans at the RHD.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ledipasvir / sofosbuvir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ledipasvir / sofosbuvir during labor and delivery.
Nursing Mothers
Risk Summary
- It is not known whether Ledipasvir or Sofosbuvir, the components of Ledipasvir/Sofosbuvir, or their metabolites are present in human breast milk, affect human milk production or have effects on the breastfed infant. When administered to lactating rats, Ledipasvir was detected in the plasma of nursing pups likely due to the presence of Ledipasvir in milk, without clear effects on nursing pups. The predominant circulating metabolite of Sofosbuvir (GS-331007) was the primary component observed in the milk of lactating rats, without effect on nursing pups.
- The development and health benefits of breastfeeding should be considered along with the mother's clinical need for Ledipasvir/Sofosbuvir and any potential adverse effects on the breastfed child from Ledipasvir/Sofosbuvir or from the underlying maternal condition.
- If Ledipasvir/Sofosbuvir is administered with ribavirin, the nursing mother's information for ribavirin also applies to this combination regimen. Refer to the ribavirin prescribing information for more information on use during lactation.
Data
- Ledipasvir: No effects of Ledipasvir on growth and postnatal development were observed in nursing pups at the highest dose tested in rats. Maternal systemic exposure (AUC) to Ledipasvir was approximately 5 times the exposure in humans at the RHD. Although not measured directly, Ledipasvir was likely present in the milk of lactating rats, since systemic exposure (AUC) to Ledipasvir of approximately 25% that of maternal exposure was observed in nursing pups on lactation day 10.
- Sofosbuvir: No effects of Sofosbuvir on growth and postnatal development were observed in nursing pups at the highest dose tested in rats. Maternal systemic exposure (AUC) to the predominant circulating metabolite of Sofosbuvir (GS-331007) was approximately 7 times the exposure in humans at the RHD, with exposure of approximately 2% that of maternal exposure observed in nursing pups on lactation day 10. In a lactation study, Sofosbuvir metabolites (primarily GS-331007) were excreted into the milk of lactating rats following administration of a single oral dose of Sofosbuvir (20 mg/kg) on lactation day 2, with milk concentrations of approximately 10% that of maternal plasma concentrations observed 1 hour post-dose.
Pediatric Use
- The safety, pharmacokinetics, and efficacy of Ledipasvir/Sofosbuvir for treatment of HCV genotype 1 infection in treatment-naïve and treatment-experienced pediatric patients 12 years of age and older without cirrhosis or with compensated cirrhosis have been established in an open-label, multicenter clinical trial (Study 1116, N=100; 80 treatment-naïve, 20 treatment-experienced) and are comparable to that observed in adults.
- The safety and efficacy of Ledipasvir/Sofosbuvir for treatment of HCV genotypes 4, 5, or 6 infection in pediatric patients 12 years of age and older or weighing at least 35 kg without cirrhosis or with compensated cirrhosis is supported by comparable Ledipasvir, Sofosbuvir, and GS-331007 exposures between adults and adolescents with HCV genotype 1 and similar efficacy and exposures across HCV genotypes 1, 4, 5, and 6 in adults.
- The safety and efficacy of Ledipasvir/Sofosbuvir have not been established in pediatric patients less than 12 years of age and weighing less than 35 kg, in pediatric patients with decompensated cirrhosis, or in pediatric liver transplant recipients.
Geriatic Use
- Clinical trials of Ledipasvir/Sofosbuvir included 225 subjects aged 65 and over (9% of total number of subjects in the clinical studies). No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. No dosage adjustment of Ledipasvir/Sofosbuvir is warranted in geriatric patients.
Gender
There is no FDA guidance on the use of Ledipasvir / sofosbuvir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ledipasvir / sofosbuvir with respect to specific racial populations.
Renal Impairment
- No dosage adjustment of Ledipasvir/Sofosbuvir is required for patients with mild or moderate renal impairment. The safety and efficacy of Ledipasvir/Sofosbuvir have not been established in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2) or ESRD requiring hemodialysis. No dosage recommendation can be given for patients with severe renal impairment or ESRD. Refer to ribavirin prescribing information regarding use in patients with renal impairment.
Hepatic Impairment
- No dosage adjustment of Ledipasvir/Sofosbuvir is required for patients with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, or C).
- Clinical and hepatic laboratory monitoring, as clinically indicated, is recommended for patients with decompensated cirrhosis receiving treatment with Ledipasvir/Sofosbuvir and ribavirin.
Females of Reproductive Potential and Males
- If Ledipasvir/Sofosbuvir is administered with ribavirin, the information for ribavirin with regard to pregnancy testing, contraception, and infertility also applies to this combination regimen. Refer to ribavirin prescribing information for additional information.
Immunocompromised Patients
There is no FDA guidance one the use of Ledipasvir / sofosbuvir in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
- Give with or without food
- Separate administration of antacids and Ledipasvir/Sofosbuvir by 4 hours.
Monitoring
- Hepatitis C virus (HCV) genotype: Prior to initiation of therapy.
- A reduction from baseline in HCV-RNA viral load may indicate efficacy.
- Hepatitis B virus (HBV) current or prior infection: Prior to initiation.
- Clinical and laboratory signs of hepatitis flare or HBV reactivation, in patients with evidence of current or prior HBV infection: During treatment and post-treatment follow-up.
- Hepatic function, as appropriate in patients with decompensated cirrhosis who are coadministered ribavirin.
IV Compatibility
There is limited information regarding the compatibility of Ledipasvir / sofosbuvir and IV administrations.
Overdosage
- No specific antidote is available for overdose with Ledipasvir/Sofosbuvir. If overdose occurs, the patient must be monitored for evidence of toxicity. Treatment of overdose with Ledipasvir/Sofosbuvir consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. Hemodialysis is unlikely to result in significant removal of Ledipasvir since Ledipasvir is highly bound to plasma protein. Hemodialysis can efficiently remove the predominant circulating metabolite of Sofosbuvir, GS-331007, with an extraction ratio of 53%.
Pharmacology
| |
| |
Ledipasvir / sofosbuvir
| |
| Combination of | |
| Ledipasvir | NS5A inhibitor |
| Sofosbuvir | NS5B (RNA polymerase) inhibitor |
| Identifiers | |
| CAS number | ? |
| ATC code | J05 |
| PubChem | |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | by mouth |
Mechanism of Action
- Ledipasvir/Sofosbuvir is a fixed-dose combination of Ledipasvir and Sofosbuvir which are direct-acting antiviral agents against the hepatitis C virus.
Structure
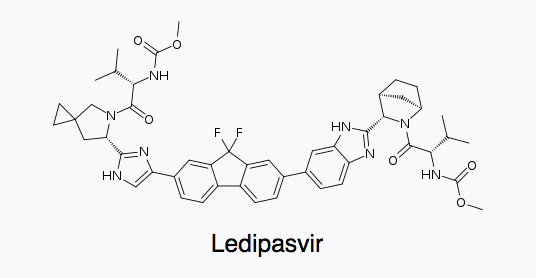
Ledipasvir

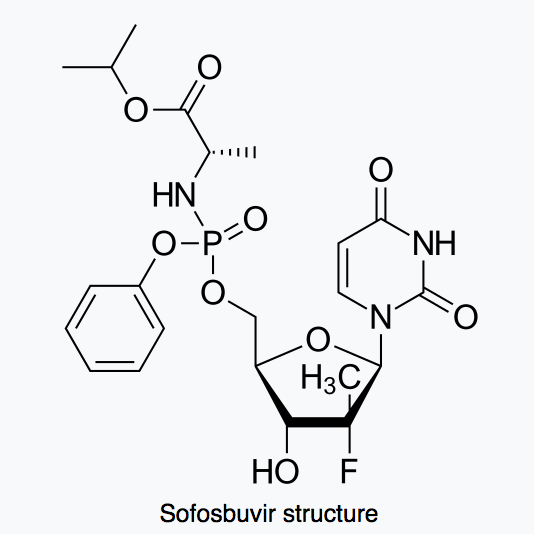
Sofosbuvir
Pharmacodynamics
Cardiac Electrophysiology
- Thorough QT studies have been conducted for Ledipasvir and Sofosbuvir.
- The effect of Ledipasvir 120 mg twice daily (2.67 times the maximum recommended dosage) for 10 days on QTc interval was evaluated in a randomized, multiple-dose, placebo-, and active-controlled (moxifloxacin 400 mg) three period crossover thorough QT trial in 59 healthy subjects. At the dose of 120 mg twice daily (2.67 times the maximum recommended dosage), Ledipasvir does not prolong QTc interval to any clinically relevant extent.
- The effect of Sofosbuvir 400 mg (maximum recommended dosage) and 1200 mg (three times the maximum recommended dosage) on QTc interval was evaluated in a randomized, single-dose, placebo-, and active-controlled (moxifloxacin 400 mg) four period crossover thorough QT trial in 59 healthy subjects. At a dose three times the maximum recommended dose, Sofosbuvir does not prolong QTc to any clinically relevant extent.
Pharmacokinetics
Absorption
- The pharmacokinetic properties of Ledipasvir, Sofosbuvir, and the predominant circulating metabolite GS-331007 have been evaluated in healthy adult subjects and in subjects with chronic hepatitis C. Following oral administration of Ledipasvir/Sofosbuvir, Ledipasvir median peak concentrations were observed 4 to 4.5 hours post-dose. Sofosbuvir was absorbed quickly and the peak median plasma concentration was observed ~0.8 to 1 hour post-dose. Median peak plasma concentration of GS-331007 was observed between 3.5 to 4 hours post-dose.
- Based on the population pharmacokinetic analysis in HCV-infected subjects, geometric mean steady-state AUC0–24 for Ledipasvir (N=2113), Sofosbuvir (N=1542), and GS-331007 (N=2113) were 7290, 1320, and 12,000 ng∙hr/mL, respectively. Steady-state Cmax for Ledipasvir, Sofosbuvir, and GS-331007 were 323, 618, and 707 ng/mL, respectively. Sofosbuvir and GS-331007 AUC0–24 and Cmax were similar in healthy adult subjects and subjects with HCV infection. Relative to healthy subjects (N=191), Ledipasvir AUC0–24 and Cmax were 24% lower and 32% lower, respectively, in HCV-infected subjects.
Effect of Food
- Relative to fasting conditions, the administration of a single dose of Ledipasvir/Sofosbuvir with a moderate fat (~600 kcal, 25% to 30% fat) or high fat (~1000 kcal, 50% fat) meal increased Sofosbuvir AUC0–inf by approximately 2-fold, but did not significantly affect Sofosbuvir Cmax. The exposures of GS-331007 and Ledipasvir were not altered in the presence of either meal type. The response rates in Phase 3 trials were similar in HCV-infected subjects who received Ledipasvir/Sofosbuvir with food or without food. Ledipasvir/Sofosbuvir can be administered without regard to food.
Distribution
- Ledipasvir is greater than 99.8% bound to human plasma proteins. After a single 90 mg dose of [14C]-Ledipasvir in healthy subjects, the blood to plasma ratio of 14C-radioactivity ranged between 0.51 and 0.66.
- Sofosbuvir is approximately 61–65% bound to human plasma proteins and the binding is independent of drug concentration over the range of 1 microgram/mL to 20 microgram/mL. Protein binding of GS-331007 was minimal in human plasma. After a single 400 mg dose of [14C]-Sofosbuvir in healthy subjects, the blood to plasma ratio of 14C-radioactivity was approximately 0.7.
Metabolism
- In vitro, no detectable metabolism of Ledipasvir was observed by human CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Evidence of slow oxidative metabolism via an unknown mechanism has been observed. Following a single dose of 90 mg [14C]-Ledipasvir, systemic exposure was almost exclusively to the parent drug (greater than 98%). Unchanged Ledipasvir is the major species present in feces.
- Sofosbuvir is extensively metabolized in the liver to form the pharmacologically active nucleoside analog triphosphate GS-461203. The metabolic activation pathway involves sequential hydrolysis of the carboxyl ester moiety catalyzed by human cathepsin A (CatA) or carboxylesterase 1 (CES1) and phosphoramidate cleavage by histidine triad nucleotide-binding protein 1 (HINT1) followed by phosphorylation by the pyrimidine nucleotide biosynthesis pathway. Dephosphorylation results in the formation of nucleoside metabolite GS-331007 that cannot be efficiently rephosphorylated and lacks anti-HCV activity in vitro. After a single 400 mg oral dose of [14C]-Sofosbuvir, GS-331007 accounted for approximately greater than 90% of total systemic exposure.
Elimination
- Following a single 90 mg oral dose of [14C]-Ledipasvir, mean total recovery of the [14C]-radioactivity in feces and urine was approximately 87%, with most of the radioactive dose recovered from feces (approximately 86%). Unchanged Ledipasvir excreted in feces accounted for a mean of 70% of the administered dose and the oxidative metabolite M19 accounted for 2.2% of the dose. These data indicate that biliary excretion of unchanged Ledipasvir is a major route of elimination, with renal excretion being a minor pathway (approximately 1%). The median terminal half-life of Ledipasvir following administration of Ledipasvir/Sofosbuvir was 47 hours.
- Following a single 400 mg oral dose of [14C]-Sofosbuvir, mean total recovery of the dose was greater than 92%, consisting of approximately 80%, 14%, and 2.5% recovered in urine, feces, and expired air, respectively. The majority of the Sofosbuvir dose recovered in urine was GS-331007 (78%) while 3.5% was recovered as Sofosbuvir. These data indicate that renal clearance is the major elimination pathway for GS-331007. The median terminal half-lives of Sofosbuvir and GS-331007 following administration of Ledipasvir/Sofosbuvir were 0.5 and 27 hours, respectively.
Specific Populations
- Race: Population pharmacokinetics analysis in HCV-infected subjects indicated that race had no clinically relevant effect on the exposure of Ledipasvir, Sofosbuvir, and GS-331007.
- Gender: Population pharmacokinetics analysis in HCV-infected subjects indicated that gender had no clinically relevant effect on the exposure of Sofosbuvir and GS-331007. AUC and Cmax of Ledipasvir were 77% and 58% higher, respectively, in females than males; however, the relationship between gender and Ledipasvir exposures was not considered clinically relevant, as high response rates (SVR12 >90%) were achieved in male and female subjects across the Phase 3 studies and the safety profiles are similar in females and males.
- Pediatric Patients: The pharmacokinetics of Ledipasvir, Sofosbuvir, and GS-331007 were determined in 100 pediatric subjects 12 years of age and older infected with HCV genotype 1 receiving a daily dose of Ledipasvir/Sofosbuvir (90 mg Ledipasvir and 400 mg Sofosbuvir). The pharmacokinetic properties of Ledipasvir, Sofosbuvir, and GS-331007 in pediatric subjects 12 years of age and older are provided in Table 6. Exposures in pediatric subjects were similar to those observed in adults.

- The pharmacokinetics of Ledipasvir or Sofosbuvir have not been established in pediatric patients less than 12 years of age.
- Geriatric Patients: Population pharmacokinetic analysis in HCV-infected subjects showed that within the age range (18 to 80 years) analyzed, age did not have a clinically relevant effect on the exposure to Ledipasvir, Sofosbuvir, and GS-331007.
- Patients with Renal Impairment: The pharmacokinetics of Ledipasvir were studied with a single dose of 90 mg Ledipasvir in HCV negative subjects with severe renal impairment (eGFR less than 30 mL/min by Cockcroft-Gault). No clinically relevant differences in Ledipasvir pharmacokinetics were observed between healthy subjects and subjects with severe renal impairment.
- The pharmacokinetics of Sofosbuvir were studied in HCV negative subjects with mild (eGFR between 50 to less than 80 mL/min/1.73 m2), moderate (eGFR between 30 to less than 50 mL/min/1.73 m2), severe renal impairment (eGFR less than 30 mL/min/1.73 m2), and subjects with ESRD requiring hemodialysis following a single 400 mg dose of sofosbuvir. Relative to subjects with normal renal function (eGFR greater than 80 mL/min/1.73 m2), the Sofosbuvir AUC0–inf was 61%, 107%, and 171% higher in mild, moderate, and severe renal impairment, while the GS-331007 AUC0–inf was 55%, 88%, and 451% higher, respectively. In subjects with ESRD, relative to subjects with normal renal function, Sofosbuvir and GS-331007 AUC0–inf was 28% and 1280% higher when Sofosbuvir was dosed 1 hour before hemodialysis compared with 60% and 2070% higher when Sofosbuvir was dosed 1 hour after hemodialysis, respectively. A 4 hour hemodialysis session removed approximately 18% of administered dose.
- Patients with Hepatic Impairment: The pharmacokinetics of Ledipasvir were studied with a single dose of 90 mg Ledipasvir in HCV negative subjects with severe hepatic impairment (Child-Pugh Class C). Ledipasvir plasma exposure (AUC0–inf) was similar in subjects with severe hepatic impairment and control subjects with normal hepatic function. Population pharmacokinetics analysis in HCV-infected subjects indicated that cirrhosis (including decompensated cirrhosis) had no clinically relevant effect on the exposure of Ledipasvir.
- The pharmacokinetics of Sofosbuvir were studied following 7-day dosing of 400 mg Sofosbuvir in HCV-infected subjects with moderate and severe hepatic impairment (Child-Pugh Class B and C). Relative to subjects with normal hepatic function, the Sofosbuvir AUC0–24 were 126% and 143% higher in moderate and severe hepatic impairment, while the GS-331007 AUC0–24 were 18% and 9% higher, respectively. Population pharmacokinetics analysis in HCV-infected subjects indicated that cirrhosis (including decompensated cirrhosis) had no clinically relevant effect on the exposure of Sofosbuvir and GS-331007.
Drug Interaction Studies
- Ledipasvir and Sofosbuvir are substrates of drug transporters P-gp and BCRP while GS-331007 is not. P-gp inducers (e.g., rifampin or St. John's wort) may decrease Ledipasvir and Sofosbuvir plasma concentrations, leading to reduced therapeutic effect of Ledipasvir/Sofosbuvir, and the use with P-gp inducers is not recommended with Ledipasvir/Sofosbuvir. Coadministration with drugs that inhibit P-gp and/or BCRP may increase Ledipasvir and Sofosbuvir plasma concentrations without increasing GS-331007 plasma concentration; Ledipasvir/Sofosbuvir may be coadministered with P-gp and/or BCRP inhibitors. Neither Ledipasvir nor Sofosbuvir is a substrate for hepatic uptake transporters OCT1, OATP1B1, or OATP1B3. GS-331007 is not a substrate for renal transporters, including organic anion transporter OAT1 or OAT3, or organic cation transporter OCT2.
- Ledipasvir is subject to slow oxidative metabolism via an unknown mechanism. In vitro, no detectable metabolism of Ledipasvir by CYP enzymes has been observed. Biliary excretion of unchanged Ledipasvir is a major route of elimination. Sofosbuvir is not a substrate for CYP and UGT1A1 enzymes. Clinically significant drug interactions with Ledipasvir/Sofosbuvir mediated by CYP or UGT1A1 enzymes are not expected.
- The effects of coadministered drugs on the exposure of Ledipasvir, Sofosbuvir, and GS-331007 are shown in Table 7.

- No effect on the pharmacokinetic parameters of Ledipasvir, Sofosbuvir, and GS-331007 was observed with raltegravir and the combination of abacavir and lamivudine; emtricitabine, rilpivirine, and tenofovir disoproxil fumarate; or dolutegravir, emtricitabine, and tenofovir disoproxil fumarate.
- Ledipasvir is an inhibitor of drug transporter P-gp and breast cancer resistance protein (BCRP) and may increase intestinal absorption of coadministered substrates for these transporters. Ledipasvir is an inhibitor of transporters OATP1B1, OATP1B3, and BSEP only at concentrations exceeding those achieved in clinic. Ledipasvir is not an inhibitor of transporters MRP2, MRP4, OCT2, OAT1, OAT3, MATE1, and OCT1. The drug-drug interaction potential of Ledipasvir is primarily limited to the intestinal inhibition of P-gp and BCRP. Clinically relevant transporter inhibition by Ledipasvir in the systemic circulation is not expected due to its high protein binding. Sofosbuvir and GS-331007 are not inhibitors of drug transporters P-gp, BCRP, MRP2, BSEP, OATP1B1, OATP1B3, and OCT1, and GS-331007 is not an inhibitor of OAT1, OCT2, and MATE1.
- Ledipasvir, Sofosbuvir, and GS-331007 are not inhibitors or inducers of CYP or UGT1A1 enzymes.
- The effects of Ledipasvir or Sofosbuvir on the exposure of coadministered drugs are shown in Table 8.

- No effect on the pharmacokinetic parameters of the following coadministered drugs was observed with Ledipasvir or Sofosbuvir: abacavir, cyclosporine, darunavir/ritonavir, dolutegravir, efavirenz, emtricitabine, lamivudine, methadone, or rilpivirine.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
- Ledipasvir: Ledipasvir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes, and in vivo rat micronucleus assays.
- Ledipasvir was not carcinogenic in a 6-month rasH2 transgenic mouse study (up to 300 mg/kg/day). Similarly, Ledipasvir was not carcinogenic in a 2-year rat study (up to 100 mg/kg/day in males and 30 mg/kg/day in females), resulting in exposures approximately 10 and 4 times, respectively, higher than the exposure in humans at the recommended human dose (RHD).
- Sofosbuvir: Sofosbuvir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes, and in vivo mouse micronucleus assays.
- Sofosbuvir was not carcinogenic in a 2-year mouse study (up to 200 mg/kg/day in males and 600 mg/kg/day in females) and in a 2-year rat study (up to 750 mg/kg/day), resulting in exposures of the predominant circulating metabolite GS-331007 of approximately 4 and 18 times (in mice) and 8 and 10 times (in rats), in males and females respectively, the exposure in humans at the RHD.
Impairment of Fertility
- Ledipasvir: Ledipasvir had no adverse effects on mating and fertility. In female rats, the mean number of corpora lutea and implantation sites were reduced slightly at maternal exposures approximately 3 times the exposure in humans at the RHD. At the highest dose levels without effects, exposures of Ledipasvir were approximately 5 and 2 times, in males and females, respectively, the exposure in humans at the RHD.
- Sofosbuvir: Sofosbuvir had no effects on embryo-fetal viability or on fertility when evaluated in rats. At the highest dose tested, exposure to the predominant circulating metabolite GS-331007 was approximately 5 times the exposure in humans at the RHD.
Clinical Studies
Description of Clinical Trials
- The efficacy and safety of Ledipasvir/Sofosbuvir were evaluated in four trials in genotype 1 HCV mono-infected subjects including one trial exclusively in treatment-experienced subjects with compensated cirrhosis (Child-Pugh A), one trial in genotype 1 or 4 HCV/HIV-1 coinfected subjects, two trials in genotype 4, 5, or 6 HCV mono-infected subjects, two trials in genotype 1 or 4 HCV infected pretransplant subjects with decompensated cirrhosis (Child-Pugh B and C) or post-transplant with Metavir F0–F3 fibrosis, compensated cirrhosis, decompensated cirrhosis, or fibrosing cholestatic hepatitis (FCH), and one trial in genotype 1 HCV pediatric subjects 12 years of age and older without cirrhosis or with compensated cirrhosis, as summarized in Table 9.

- Ledipasvir/Sofosbuvir was administered once daily by mouth in these trials. For subjects without cirrhosis or with compensated cirrhosis who received ribavirin (RBV), the RBV dosage was 1000 mg per day for subjects weighing less than 75 kg or 1200 mg per day for subjects weighing at least 75 kg. For subjects with decompensated cirrhosis in SOLAR-1 and SOLAR-2 studies, the starting RBV dosage was 600 mg per day regardless of transplantation status. RBV dose adjustments were performed according to the RBV labeling.
- Serum HCV RNA values were measured during the clinical trials using the COBAS TaqMan HCV test (version 2.0), for use with the High Pure System in ION-3, ION-1, ION-2, SIRIUS, and ION-4 studies or the COBAS AmpliPrep/COBAS Taqman HCV test (version 2.0) in ELECTRON-2, 1119, SOLAR-1, SOLAR-2, and 1116 studies. The COBAS TaqMan HCV test (version 2.0) for use with the High Pure System has a lower limit of quantification (LLOQ) of 25 IU per mL and the COBAS AmpliPrep/COBAS Taqman HCV test (version 2.0) has a LLOQ of 15 IU per mL. Sustained virologic response (SVR12), defined as HCV RNA less than LLOQ at 12 weeks after the cessation of treatment, was the primary endpoint in studies in adults and the key efficacy endpoint in the study in pediatric subjects 12 years of age and older. Relapse was a secondary endpoint, which was defined as HCV RNA greater than or equal to LLOQ with 2 consecutive values or last available post-treatment measurement during the post-treatment period after achieving HCV RNA less than LLOQ at end of treatment.
Clinical Trials in Subjects with Genotype 1 HCV
Treatment-Naïve Adults without Cirrhosis ─ ION-3 (Study 0108)
- ION-3 was a randomized, open-label trial in treatment-naïve non-cirrhotic subjects with genotype 1 HCV. Subjects were randomized in a 1:1:1 ratio to one of the following three treatment groups and stratified by HCV genotype (1a vs 1b): Ledipasvir/Sofosbuvir for 8 weeks, Ledipasvir/Sofosbuvir for 12 weeks, or Ledipasvir/Sofosbuvir + ribavirin for 8 weeks.
- Demographics and baseline characteristics were balanced across the treatment groups. Of the 647 treated subjects, the median age was 55 years (range: 20 to 75); 58% of the subjects were male; 78% were White; 19% were Black; 6% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 18 to 56 kg/m2); 81% had baseline HCV RNA levels greater than or equal to 800,000 IU per mL; 80% had genotype 1a HCV infection; 73% had non-C/C IL28B alleles (CT or TT).
- Table 10 presents the SVR12 for the Ledipasvir/Sofosbuvir treatment groups in the ION-3 trial after 8 and 12 weeks of Ledipasvir/Sofosbuvir treatment. Ribavirin was not shown to increase the SVR12 observed with Ledipasvir/Sofosbuvir. Therefore, the Ledipasvir/Sofosbuvir + ribavirin arm is not presented in Table 10.

- The treatment difference between the 8-week treatment of Ledipasvir/Sofosbuvir and 12-week treatment of Ledipasvir/Sofosbuvir was –2.3% (97.5% confidence interval –7.2% to 2.5%). Among subjects with a baseline HCV RNA less than 6 million IU per mL, the SVR12 was 97% (119/123) with 8-week treatment of Ledipasvir/Sofosbuvir and 96% (126/131) with 12-week treatment of Ledipasvir/Sofosbuvir.
- Relapse rates by baseline viral load are presented in Table 11.

Treatment-Naïve Adults with or without Cirrhosis ─ ION-1 (Study 0102)
- ION-1 was a randomized, open-label trial that evaluated 12 and 24 weeks of treatment with Ledipasvir/Sofosbuvir with or without ribavirin in 865 treatment-naïve subjects with genotype 1 HCV including those with cirrhosis. Subjects were randomized in a 1:1:1:1 ratio to receive Ledipasvir/Sofosbuvir for 12 weeks, Ledipasvir/Sofosbuvir + ribavirin for 12 weeks, Ledipasvir/Sofosbuvir for 24 weeks, or Ledipasvir/Sofosbuvir + ribavirin for 24 weeks. Randomization was stratified by the presence or absence of cirrhosis and HCV genotype (1a vs 1b).
- Demographics and baseline characteristics were balanced across the treatment groups. Of the 865 treated subjects, the median age was 54 years (range: 18 to 80); 59% of the subjects were male; 85% were White; 12% were Black; 12% were Hispanic or Latino; mean body mass index was 27 kg/m2 (range: 18 to 48 kg/m2); 79% had baseline HCV RNA levels greater than or equal to 800,000 IU per mL; 67% had genotype 1a HCV infection; 70% had non-C/C IL28B alleles (CT or TT); and 16% had cirrhosis.
- Table 12 presents the SVR12 for the treatment group of Ledipasvir/Sofosbuvir for 12 weeks in the ION-1 trial. Ribavirin was not shown to increase SVR12 observed with Ledipasvir/Sofosbuvir. Therefore, the Ledipasvir/Sofosbuvir + ribavirin arm is not presented in Table 12.

- SVR12 for selected subgroups are presented in Table 13.

Previously-Treated Adults with or without Cirrhosis ─ ION-2 (Study 0109)
- ION-2 was a randomized, open-label trial that evaluated 12 and 24 weeks of treatment with Ledipasvir/Sofosbuvir with or without ribavirin in genotype 1 HCV-infected subjects with or without cirrhosis who failed prior therapy with an interferon-based regimen, including regimens containing an HCV protease inhibitor. Subjects were randomized in a 1:1:1:1 ratio to receive Ledipasvir/Sofosbuvir for 12 weeks, Ledipasvir/Sofosbuvir + ribavirin for 12 weeks, Ledipasvir/Sofosbuvir for 24 weeks, or Ledipasvir/Sofosbuvir + ribavirin for 24 weeks. Randomization was stratified by the presence or absence of cirrhosis, HCV genotype (1a vs 1b) and response to prior HCV therapy (relapse/breakthrough vs nonresponse).
- Demographics and baseline characteristics were balanced across the treatment groups. Of the 440 treated subjects, the median age was 57 years (range: 24 to 75); 65% of the subjects were male; 81% were White; 18% were Black; 9% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 19 to 50 kg/m2); 89% had baseline HCV RNA levels greater than or equal to 800,000 IU per mL; 79% had genotype 1a HCV infection; 88% had non-C/C IL28B alleles (CT or TT); and 20% had cirrhosis. Forty-seven percent (47%) of the subjects failed a prior therapy of pegylated interferon and ribavirin. Among these subjects, 49% were relapse/breakthrough and 51% were non-responder. Fifty-three percent (53%) of the subjects failed a prior therapy of pegylated interferon and ribavirin with an HCV protease inhibitor. Among these subjects, 62% were relapse/breakthrough and 38% were non-responder.
- Table 14 presents the SVR12 for the Ledipasvir/Sofosbuvir treatment groups in the ION-2 trial. Ribavirin was not shown to increase SVR12 observed with Ledipasvir/Sofosbuvir. Therefore, the Ledipasvir/Sofosbuvir + ribavirin arms are not presented in Table 14.

- Among the subjects with available SVR12 and SVR24 data (206/218), all subjects who achieved SVR12 in the ION-2 study also achieved SVR24.
- SVR12 and relapse rates for selected subgroups are presented in Tables 15 and 16.


Previously-Treated Adults with Cirrhosis ─ SIRIUS (Study 0121)
- SIRIUS was a randomized, double-blind and placebo-controlled trial that evaluated the efficacy of Ledipasvir/Sofosbuvir + ribavirin for 12 weeks or Ledipasvir/Sofosbuvir without ribavirin for 24 weeks in genotype 1 HCV-infected subjects with compensated cirrhosis who failed prior therapy with a Peg-IFN + RBV regimen followed by a subsequent Peg-IFN + RBV + an HCV protease inhibitor regimen. Subjects were randomized in a 1:1 ratio to receive placebo for 12 weeks followed by Ledipasvir/Sofosbuvir + ribavirin for 12 weeks or Ledipasvir/Sofosbuvir for 24 weeks. Randomization was stratified by HCV genotype (1a vs 1b) and response to prior HCV therapy (never achieved HCV RNA less than LLOQ vs achieved HCV RNA less than LLOQ).
- Demographics and baseline characteristics were balanced across the treatment groups. Of the 155 randomized subjects, the median age was 56 years (range: 23 to 77); 74% of the subjects were male; 97% were White; mean body mass index was 27 kg/m2 (range: 19 to 47 kg/m2); 63% had genotype 1a HCV infection; 94% had non-C/C IL28B alleles (CT or TT). One subject discontinued therapy while on placebo, and was not included in the efficacy analysis.
- The SVR12 was 96% (74/77) and 97% (75/77) in subjects treated with Ledipasvir/Sofosbuvir + ribavirin for 12 weeks and Ledipasvir/Sofosbuvir for 24 weeks without ribavirin, respectively. All 5 subjects who did not achieve SVR12 relapsed.
Clinical Trials in Subjects with Genotype 4, 5, or 6 HCV
- Below are trial descriptions, SVR12 and relapse data in the genotype 4, 5, and 6 HCV populations. Trial results in the genotype 4, 5, and 6 HCV populations are based upon limited number of subjects in some subgroups, particularly in subjects who have been previously-treated and subjects with cirrhosis.
Genotype 4
- In two open-label studies (Study 1119 and ION-4), Ledipasvir/Sofosbuvir was administered for 12 weeks to treatment-naïve and previously-treated adult subjects with genotype 4 HCV infection. Study 1119 enrolled 44 treatment-naïve or previously-treated subjects with genotype 4 HCV, with or without cirrhosis. ION-4 enrolled 4 treatment-naïve and 4 previously-treated subjects with genotype 4 HCV infection who were coinfected with HIV-1, none of whom had cirrhosis.
- In Study 1119, the overall SVR12 rate was 93% (41/44). SVR12 was similar based upon prior HCV treatment history and cirrhosis status. In ION-4, all 8 subjects achieved SVR12.
Genotype 5
- In the open-label 1119 trial, Ledipasvir/Sofosbuvir was administered for 12 weeks to 41 treatment-naïve or previously-treated adult subjects with genotype 5 HCV infection, with or without cirrhosis. The overall SVR12 was 93% (38/41). SVR12 was similar based upon prior HCV treatment history and cirrhosis status.
Genotype 6
- In the open-label ELECTRON-2 trial, Ledipasvir/Sofosbuvir was administered for 12 weeks to 25 treatment-naïve or previously-treated adult subjects with genotype 6 HCV infection, with or without cirrhosis. The overall SVR12 was 96% (24/25). SVR12 was similar based upon prior HCV treatment history and cirrhosis status. The single subject who relapsed discontinued study treatment early (at approximately Week 8).
Clinical Trials in Subjects Coinfected with HCV and HIV-1
- ION-4 was an open-label clinical trial that evaluated the safety and efficacy of 12 weeks of treatment with Ledipasvir/Sofosbuvir without ribavirin in HCV treatment-naïve and previously-treated adult subjects with genotype 1 or 4 HCV infection who were coinfected with HIV-1. Treatment-experienced subjects had failed prior treatment with Peg-IFN + RBV, Peg-IFN + RBV + an HCV protease inhibitor or Sofosbuvir + RBV. Subjects were on a stable HIV-1 antiretroviral therapy that included emtricitabine + tenofovir disoproxil fumarate, administered with efavirenz, rilpivirine, or raltegravir.
- Of the 335 treated subjects, the median age was 52 years (range: 26 to 72); 82% of the subjects were male; 61% were White; 34% were Black; mean body mass index was 27 kg/m2 (range: 18 to 66 kg/m2); 75% had genotype 1a HCV infection; 2% had genotype 4 infection; 76% had non-C/C IL28B alleles (CT or TT); and 20% had compensated cirrhosis. Fifty-five percent (55%) of the subjects were treatment-experienced.
- Table 17 presents the SVR12 in the ION-4 trial after 12 weeks of Ledipasvir/Sofosbuvir treatment.

- SVR12 rates were 94% (63/67) in subjects with cirrhosis and 98% (46/47) in subjects who were previously-treated and had cirrhosis. The relapse rate in the ION-4 trial in Black subjects was 9% (10/115), all of whom were IL28B non-CC genotype, and none in non-Black subjects (0/220). In the ION-1, ION-2, and ION-3 HCV mono-infection studies, relapse rates were 3% (10/305) in Black subjects and 2% (26/1637) in non-Black subjects.
- No subject had HIV-1 rebound during the study. The percentage of CD4+ cells did not change during treatment. Median CD4+ cell count increase of 29 cells/mm3 was observed at the end of treatment with Ledipasvir/Sofosbuvir for 12 weeks.
Clinical Trials in Liver Transplant Recipients and/or Subjects with Decompensated Cirrhosis
- SOLAR-1 and SOLAR-2 were two open-label trials that evaluated 12 and 24 weeks of treatment with Ledipasvir/Sofosbuvir in combination with ribavirin in HCV treatment-naïve and previously-treated adult subjects with genotype 1 and 4 infection who had undergone liver transplantation and/or who had decompensated liver disease. The two trials were identical in study design. Subjects were enrolled in one of the seven groups in the trials based on liver transplantation status and severity of hepatic impairment (see TABLE 16). Subjects with a CPT score greater than 12 were excluded. Within each group, subjects were randomized in a 1:1 ratio to receive Ledipasvir/Sofosbuvir + RBV for 12 weeks or Ledipasvir/Sofosbuvir + RBV for 24 weeks. For subjects with decompensated cirrhosis in SOLAR-1 and SOLAR-2 studies, the starting RBV dosage was 600 mg per day regardless of transplantation status. RBV dose adjustments were performed according to the RBV labeling.
- Demographics and baseline characteristics were balanced across the treatment groups. Of the 670 treated subjects, the median age was 59 years (range: 21 to 81); 77% of the subjects were male; 91% were White; mean body mass index was 28 kg/m2 (range: 18 to 49 kg/m2); 94% and 6% had genotype 1 and 4 HCV infection, respectively; 78% of the subjects failed a prior HCV therapy.
- Table 18 presents the pooled SVR12 rates for SOLAR-1 and SOLAR-2 in subjects with genotype 1 HCV treated with Ledipasvir/Sofosbuvir + RBV for 12 weeks. The SVR12 rates observed with 24 weeks of Ledipasvir/Sofosbuvir + RBV were similar to the SVR12 rates observed with 12 weeks of treatment. Therefore, the results for the Ledipasvir/Sofosbuvir + RBV 24 weeks arm are not presented in Table 18.

- There were 7 subjects with fibrosing cholestatic hepatitis in the 12 week treatment arm, and all subjects achieved SVR12.
- In genotype 4 HCV post-transplant subjects without cirrhosis or with compensated cirrhosis treated with Ledipasvir/Sofosbuvir + RBV for 12 weeks (N=12), the SVR12 rate was similar to rates reported with genotype 1; no subjects relapsed. Available data in subjects with genotype 4 HCV who had decompensated cirrhosis (pre- and post-liver transplantation) were insufficient for dosing recommendations; therefore, these results are not presented.
Clinical Trial in Pediatric Subjects
- The efficacy of Ledipasvir/Sofosbuvir was evaluated in an open-label trial (Study 1116) that evaluated 12 weeks of treatment with Ledipasvir/Sofosbuvir once daily in genotype 1 HCV treatment-naïve (N=80) and treatment-experienced (N=20) pediatric subjects 12 years of age and older without cirrhosis or with compensated cirrhosis.
- Demographics and baseline characteristics were balanced across treatment-naïve and treatment-experienced subjects (patients had failed an interferon based regimen with or without ribavirin). Of the 100 treated subjects, the median age was 15 years (range: 12 to 17); 63% of the subjects were female; 90% were White, 7% were Black, and 2% were Asian; 13% were Hispanic/Latino; mean body mass index was 23 kg/m2 (range: 13.1 to 36.6 kg/m2); mean weight was 61 kg (range 33 to 126 kg); 55% had baseline HCV RNA levels greater than or equal to 800,000 IU/mL; 81% had genotype 1a HCV infection; 76% had non-CC IL28B alleles (CT or TT). One subject had known compensated cirrhosis. The majority of subjects (84%) had been infected through vertical transmission.
- The SVR12 rate was 98% overall (98% [78/80] in treatment-naïve subjects and 100% [20/20] in treatment-experienced subjects). No subject experienced on-treatment virologic failure or relapse. Two subjects were lost to follow-up.
How Supplied
- Ledipasvir/Sofosbuvir tablets are orange, diamond-shaped, film-coated, debossed with "GSI" on one side and "7985" on the other side of the tablet. Each bottle contains 28 tablets (NDC 61958-1801-1), a silica gel desiccant, polyester coil, and is closed with a child-resistant closure.
Storage
- Store at room temperature below 30 °C (86 °F).
- Dispense only in original container.
- Do not use if seal over bottle opening is broken or missing.
Images
Drug Images
{{#ask: Page Name::Ledipasvir / sofosbuvir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Ledipasvir / sofosbuvir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
- Inform patients that HBV reactivation can occur in patients coinfected with HBV during or after treatment of HCV infection. Advise patients to tell their healthcare provider if they have a history of HBV infection.
Serious Symptomatic Bradycardia When Coadministered with Amiodarone
- Advise patients to seek medical evaluation immediately for symptoms of bradycardia such as near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pain, confusion or memory problems.
Drug Interactions
- Inform patients that Ledipasvir/Sofosbuvir may interact with other drugs. Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products including St. John's wort.
Pregnancy
- Advise patients to avoid pregnancy during combination treatment with Ledipasvir/Sofosbuvir and ribavirin and for 6 months after completion of treatment. Inform patients to notify their healthcare provider immediately in the event of a pregnancy.
Administration
- Advise patients to take Ledipasvir/Sofosbuvir every day at the regularly scheduled time with or without food. Inform patients that it is important not to miss or skip doses and to take Ledipasvir/Sofosbuvir for the duration that is recommended by the physician.

Precautions with Alcohol
Alcohol-Ledipasvir / sofosbuvir interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Harvoni
Look-Alike Drug Names
There is limited information regarding Ledipasvir / sofosbuvir Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.