Ivermectin (topical)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ivermectin (topical) is an anthelmintic and anti-infective agent that is FDA approved for the treatment of head lice infestations in patients 6 months of age and older. Common adverse reactions include pruritus and urticaria.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- SKLICE™ Lotion is indicated for the topical treatment of head lice infestations in patients 6 months of age and older.
Adjunctive Measures
- SKLICE Lotion should be used in the context of an overall lice management program:
- Wash (in hot water) or dry-clean all recently worn clothing, hats, used bedding and towels.
- Wash personal care items such as combs, brushes and hair clips in hot water.
- A fine-tooth comb or special nit comb may be used to remove dead lice and nits.
Dosing Information
- For topical use only. SKLICE Lotion is not for oral, ophthalmic, or intravaginal use.
- Apply SKLICE Lotion to dry hair in an amount sufficient (up to 1 tube) to thoroughly coat the hair and scalp. Leave SKLICE Lotion on the hair and scalp for 10 minutes, and then rinse off with water.
- The tube is intended for single use; discard any unused portion.
- Avoid contact with eyes.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ivermectin (topical) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ivermectin (topical) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ivermectin (topical) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ivermectin (topical) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ivermectin (topical) in pediatric patients.
Contraindications
There is limited information regarding Ivermectin (topical) Contraindications in the drug label.
Warnings
Ingestion in Pediatric Patients
- In order to prevent ingestion, SKLICE Lotion should only be administered to pediatric patients under the direct supervision of an adult.
Adverse Reactions
Clinical Trials Experience
Clinical Trial Experience=
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- The data described below reflect exposure to a single 10 minute treatment of SKLICE Lotion in 379 patients, ages 6 months and older, in placebo-controlled trials. Of these subjects, 47 subjects were age 6 months to 4 years, 179 subjects were age 4 to 12 years, 56 subjects were age 12 to 16 years and 97 subjects were age 16 or older. Adverse reactions, reported in less than 1% of subjects treated with SKLICE Lotion, include conjunctivitis, ocular hyperemia, eye irritation, dandruff, dry skin, and skin burning sensation.
Postmarketing Experience
There is limited information regarding Ivermectin (topical) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Ivermectin (topical) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies with SKLICE Lotion in pregnant women. SKLICE Lotion should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- No comparisons of animal exposure with human exposure are provided due to the low systemic exposure noted in the clinical pharmacokinetic study [see Clinical Pharmacology (12.3)].
Human Data
- There are published reports of oral ivermectin use during human pregnancy. In an open label study, 397 women in their second trimester of pregnancy were treated with ivermectin tablets and albendazole at the labeled dose rate for soil-transmitted helminths and compared with a pregnant, non-treated population. No differences in pregnancy outcomes were observed between treated and untreated populations.
Animal Data
- Systemic embryofetal development studies were conducted in mice, rats and rabbits. Oral doses of 0.1, 0.2, 0.4, 0.8, and 1.6 mg/kg/day ivermectin were administered during the period of organogenesis (gestational days 6–15) to pregnant female mice. Maternal death occurred at 0.4 mg/kg/day and above. Cleft palate occurred in the fetuses from the 0.4, 0.8, and 1.6 mg/kg/day groups. Exencephaly was seen in the fetuses from the 0.8 mg/kg group. Oral doses of 2.5, 5, and 10 mg/kg/day ivermectin were administered during the period of organogenesis (gestational days 6–17) to pregnant female rats. Maternal death and pre-implantation loss occurred at 10 mg/kg/day. Cleft palate and wavy ribs were seen in fetuses from the 10 mg/kg/day group. Oral doses of 1.5, 3, and 6 mg/kg/day ivermectin were administered during the period of organogenesis (gestational days 6–18) to pregnant female rabbits. Maternal toxicity and abortion occurred at 6 mg/kg/day. Cleft palate and clubbed forepaws occurred in the fetuses from the 3 and 6 mg/kg groups. These teratogenic effects were found only at or near doses that were maternally toxic to the pregnant female. Therefore, ivermectin does not appear to be selectively fetotoxic to the developing fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ivermectin (topical) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ivermectin (topical) during labor and delivery.
Nursing Mothers
- Following oral administration, ivermectin is excreted in human milk in low concentrations. This has not been evaluated following topical administration. Caution should be exercised when SKLICE Lotion is administered to a nursing woman.
Pediatric Use
- The safety and effectiveness of SKLICE Lotion have been established for pediatric patients 6 months of age and older [see Clinical Pharmacology (12.3) and Clinical Studies (14)].
- The safety of SKLICE Lotion has not been established in pediatric patients below the age of 6 months. SKLICE Lotion is not recommended in pediatric patients under 6 months of age because of the potential increased systemic absorption due to a high ratio of skin surface area to body mass and the potential for an immature skin barrier and risk of ivermectin toxicity.
Geriatic Use
- Clinical studies of SKLICE Lotion did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Ivermectin (topical) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ivermectin (topical) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ivermectin (topical) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ivermectin (topical) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ivermectin (topical) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ivermectin (topical) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Ivermectin (topical) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ivermectin (topical) and IV administrations.
Overdosage
- In accidental or significant exposure to unknown quantities of veterinary formulations of ivermectin in humans, either by ingestion, inhalation, injection, or exposure to body surfaces, the following adverse effects have been reported most frequently: rash, edema, headache, dizziness, asthenia, nausea, vomiting, and diarrhea. Other adverse effects that have been reported include: seizure, ataxia, dyspnea, abdominal pain, paresthesia, urticaria, and contact dermatitis.
- In case of accidental poisoning, supportive therapy, if indicated, should include parenteral fluids and electrolytes, respiratory support (oxygen and mechanical ventilation if necessary) and pressor agents if clinically significant hypotension is present. Induction of emesis and/or gastric lavage as soon as possible, followed by purgatives and other routine anti-poison measures, may be indicated if needed to prevent absorption of ingested material.
Pharmacology
| |
Ivermectin (topical)
| |
| Systematic (IUPAC) name | |
| 22,23-dihydroavermectin B1a + 22,23-dihydroavermectin B1b | |
| Identifiers | |
| CAS number | 71827-03-7 |
| ATC code | P02 Template:ATCvet Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 875.10 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 93% |
| Metabolism | Liver (CYP450) |
| Half life | 18 hours |
| Excretion | Feces; <1% urine |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral, topical |
Mechanism of Action
- Ivermectin, a member of the avermectin class, causes death of parasites, primarily through binding selectively and with high affinity to glutamate-gated chloride channels, which occur in invertebrate nerve and muscle cells. This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA). The selective activity of compounds of this class is attributable to the fact that some mammals do not have glutamate-gated chloride channels, the avermectins have a low affinity for mammalian ligand-gated chloride channels, and ivermectin does not readily cross the blood-brain barrier in humans.
Structure
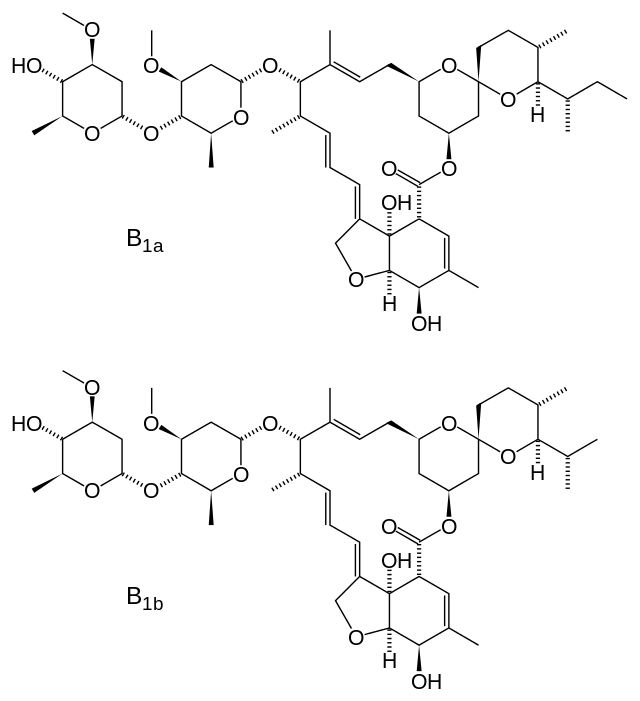
- SKLICE (ivermectin) Lotion, for topical administration, is an off-white/tan lotion containing 0.5% ivermectin.
- Ivermectin, the active ingredient, is a pediculicide, derived from the fermentation of a soil dwelling actinomycete, Streptomyces avermitilis.
- Ivermectin is a mixture containing at least 90% 5-O-demethyl-22,23-dihydroavermectin A1a and less than 10% 5-O-demethyl-25-de(1-methylpropyl)-22,23-dihydro25-(1-methylethyl) avermectin A1a, generally referred to as 22,23-dihydroavermectin B1a and B1b, or H2B1a and H2B1b, respectively. The respective empirical formulas are C48H74O14 and C47H72O14, with molecular weights of 875.10 and 861.07, respectively. The structural formulas are:

Pharmacodynamics
There is limited information regarding Ivermectin (topical) Pharmacodynamics in the drug label.
Pharmacokinetics
- The absorption of ivermectin from SKLICE Lotion was evaluated in a clinical study in subjects aged from 6 months to 3 years. This study evaluated pharmacokinetics in 20 lice infested subjects, and 13 of these subjects weighed 15 kg or less (overall weight range 8.5-23.9 kg). All enrolled subjects received a single treatment with SKLICE Lotion. The systemic ivermectin exposure was evaluated using an assay with a lower limit of quantitation of 0.05 ng/mL. The mean (± standard deviation) plasma maximum concentration (Cmax) and area under the concentration-time curve from 0 to time of last measurable concentration (AUC0-tlast) were 0.24 ± 0.23 ng/mL and 6.7 ± 11.2hr•ng/mL, respectively. These levels are much lower than those observed following oral administration of 165 mcg/kg dose of ivermectin.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of SKLICE Lotion or ivermectin.
- Ivermectin was not genotoxic in vitro in the Ames test, the mouse lymphoma assay, or the unscheduled DNA synthesis assay in human fibroblasts.
- Ivermectin had no adverse effects on fertility in rats at repeated oral doses of up to 3.6 mg/kg/day.
Clinical Studies
- Two identical multi-center, randomized, double-blind, vehicle-controlled studies were conducted in subjects 6 months of age and older with head lice infestation. All subjects received a single application of either SKLICE Lotion or vehicle control with instructions not to use a nit comb. For the evaluation of efficacy, the youngest subject from each household was considered to be the index subject of the household (N=289). Other enrolled infested household members received the same treatment as the youngest subject and were evaluated for all safety parameters [see Adverse Reactions (6.1)].
- The primary efficacy was assessed as the proportion of index subjects who were free of live lice at day 2 and through day 8 to the final evaluation 14 (+2) days following a single application. Subjects with live lice present at any time up to the final evaluation were considered treatment failures. Table 1 contains the proportion of subjects who were free of live lice in each of the two trials.

How Supplied
SKLICE Lotion, 0.5% is supplied in a 4 oz (117g) laminate tube (NDC 49281-183-71).
Storage
- Store at room temperature 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F).
- Do not freeze.
Images
Drug Images
{{#ask: Page Name::Ivermectin (topical) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Package Label - Principal Display Panel - Sklice Tube Label

Package Label - Principal Display Panel - Sklice Tube Carton

{{#ask: Label Page::Ivermectin (topical) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform the patient and caregiver of the following instructions:
- Apply SKLICE Lotion to dry scalp and dry scalp hair.
- Avoid contact with eyes.
- Do not swallow SKLICE Lotion.
- Keep out of reach of children. Use on children should be under the direct supervision of an adult.
- For single use only; do not retreat.
- Discard tube after use.
- Wash hands after applying SKLICE Lotion.




Precautions with Alcohol
Alcohol-Ivermectin (topical) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Sklice
Look-Alike Drug Names
There is limited information regarding Ivermectin (topical) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.