IMITREX tablet adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Adverse Reactions
The following adverse reactions are discussed in more detail in other sections of the prescribing information:
Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina [see Warnings and Precautions (5.1)]
Arrhythmias [see Warnings and Precautions (5.2)]
Chest, throat, neck, and/or jaw pain/tightness/pressure [see Warnings and Precautions (5.3)]
Cerebrovascular events [see Warnings and Precautions (5.4)]
Other vasospasm reactions [see Warnings and Precautions (5.5)]
Medication overuse headache [see Warnings and Precautions (5.6)]
Serotonin syndrome [see Warnings and Precautions (5.7)]
Increase in blood pressure [see Warnings and Precautions (5.8)]
Hypersensitivity reactions [see Contraindications (4) and Warnings and Precautions (5.9)]
Seizures [see Warnings and Precautions (5.10)]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 1 lists adverse reactions that occurred in placebo-controlled clinical trials in patients who took at least 1 dose of study drug. Only treatment-emergent adverse reactions that occurred at a frequency of 2% or more in any group treated with IMITREX Tablets and that occurred at a frequency greater than the placebo group are included in Table 1.
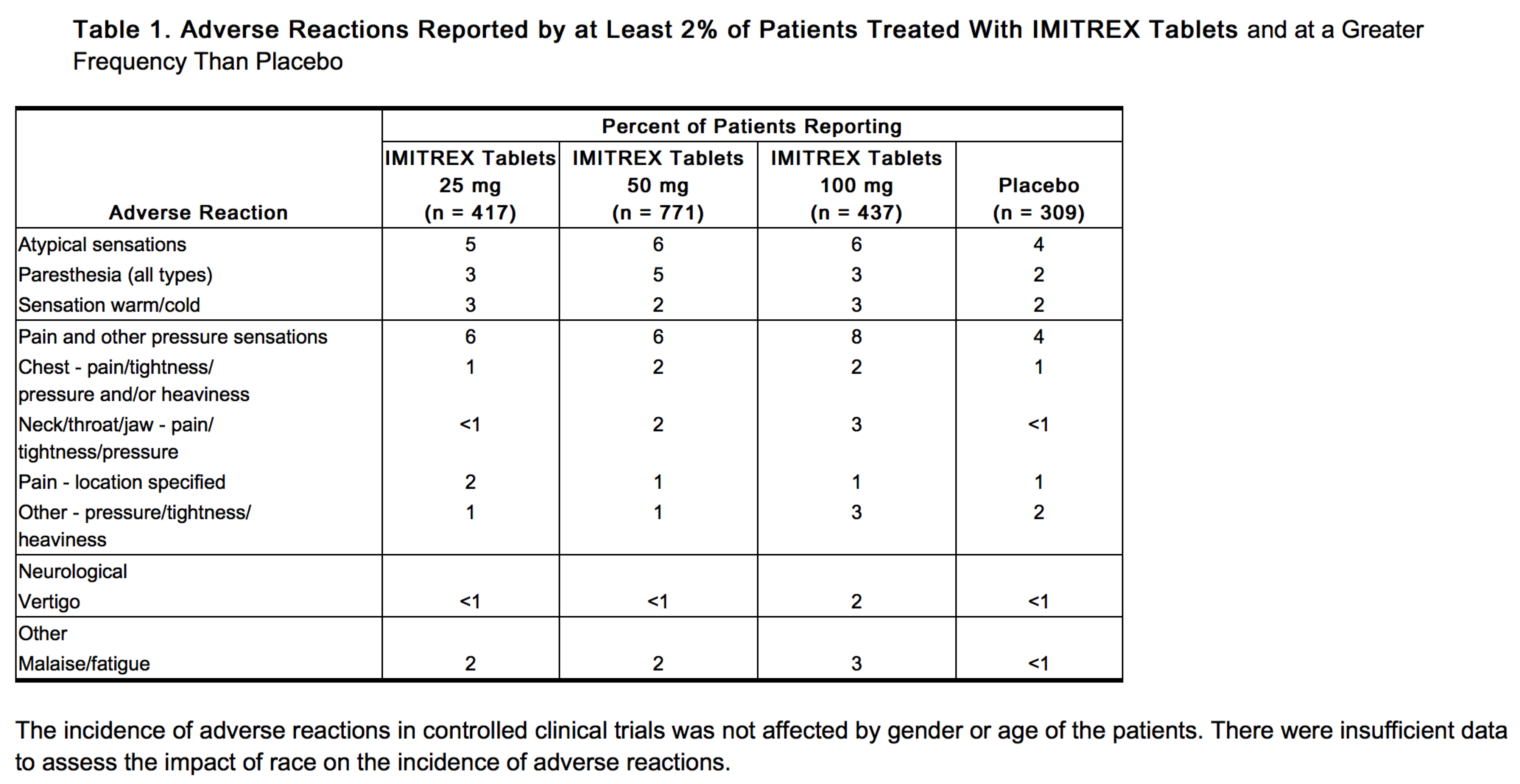 |
The incidence of adverse reactions in controlled clinical trials was not affected by gender or age of the patients. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of IMITREX Tablets, IMITREX Nasal Spray, and IMITREX Injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reactions have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to IMITREX or a combination of these factors.
Cardiovascular: Hypotension, palpitations.
Neurological: Dystonia, tremor.[1]
References
Adapted from the FDA Package Insert.