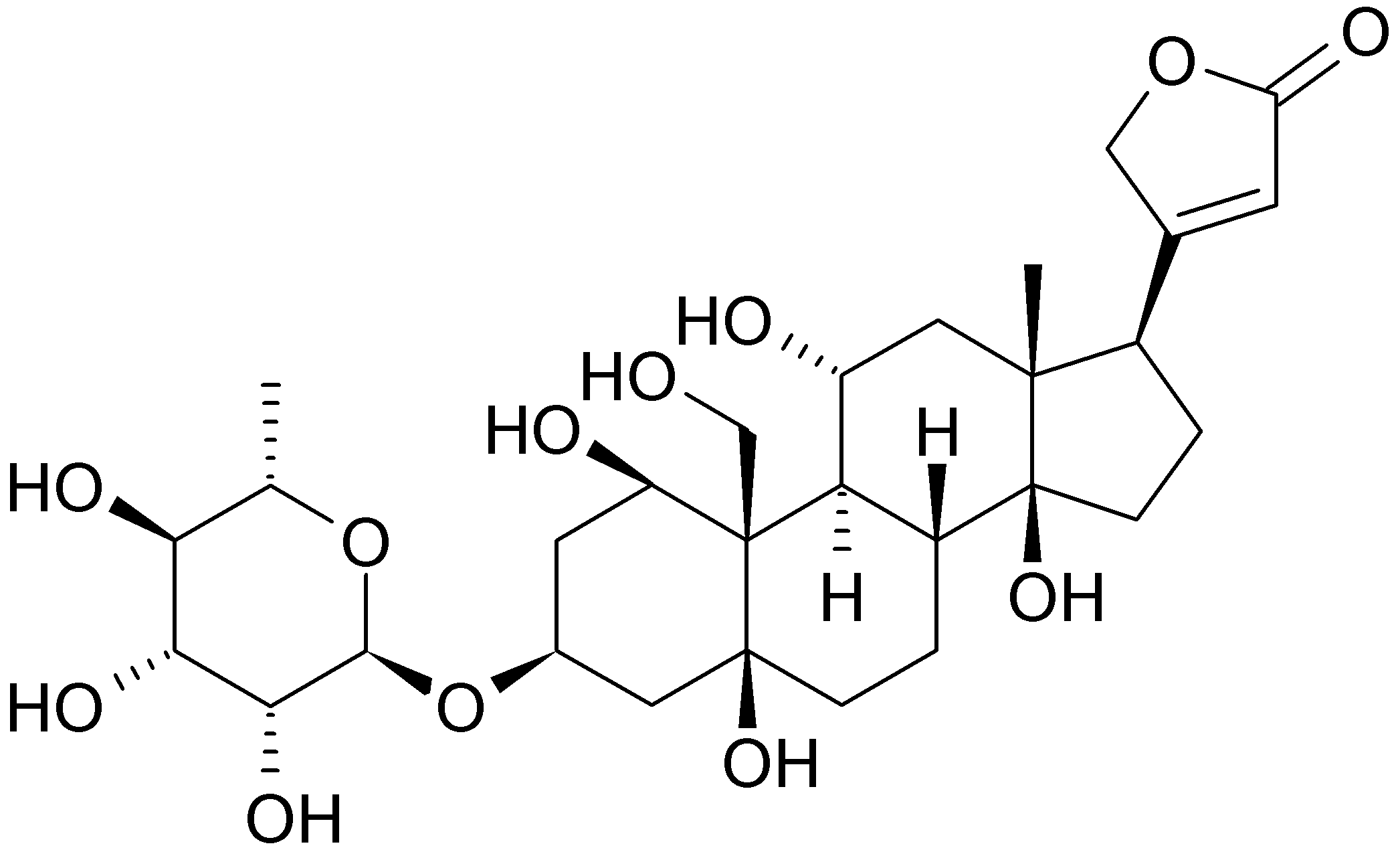
Ouabain
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C29H44O12 |
| Molar mass | 584.652 |
|
WikiDoc Resources for Ouabain |
|
Articles |
|---|
|
Most recent articles on Ouabain |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ouabain at Clinical Trials.gov Clinical Trials on Ouabain at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ouabain
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Ouabain Risk calculators and risk factors for Ouabain
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ouabain |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Ouabain (from Somali waabaayo, "arrow poison" through French ouabaïo) also known as g-strophanthin, is a cardiac glycoside. Sometimes used in the treatment of heart conditions, it has also been used as a powerful arrow poison by some tribal peoples.
Sources
Ouabain (g-strophanthin) is found in the ripe seeds of African plants Strophanthus gratus and the bark of Acokanthera ouabaio.
Function
It is well understood that the classical mechanism of action of ouabain involves its binding to and inhibition of the plasma membrane Na+/K+-ATPase (sodium/potassium pump) especially at the higher concentrations attainable in vitro or with intravenous dosage. Inhibition of the sodium pump and the secondary effect on the handling of calcium ions by sodium calcium exchanger (NCX) is widely believed to underlie the original beneficial effect as an inotropic agent following intravenous use of ouabain; digoxin is a structurally related and more lipophilic cardiac glycoside that largely replaced ouabain for therapy because of its superior oral bioavailability. Digoxin continues to be used therapeutically for many of the same indications in which ouabain was used (including atrial fibrillation and congestive heart failure).
In addition to the classical mechanism of action of ouabain at high concentrations, low (nanomolar and subnanomolar) concentrations of ouabain, as they were found endogenously (see below) or after oral intake, stimulate the Na-K-ATPase.[1] This stimulatory effect does not appear to be mimicked by digoxin.[2] Furthermore ouabain acts as a signal transducer.[3]
Endogenous ouabain and ouabain mimics
In 1991, a potent sodium pump inhibitor was isolated from the human circulation and identified as ouabain. Several additional observations led to the view that the circulating ouabain in humans was an endogenous hormone.[4] A reinterpretation of a portion of the analytical data led to the proposal that endogenous ouabain may have been the 11 epimer, i.e., an isomer of plant ouabain.[5] However, this possibility has been excluded by the synthesis of the 11 epimer and the demonstration that it has different chromatographic behavior from ouabain. There is compelling analytical evidence that ouabain is synthesized in the adrenal gland.[6] A ouabain-like factor may also be made in the hypothalamus[7] and possibly the heart where it may be stimulated by oxygen deficiency.[8] In the latter instances, analytical data are needed to support the identity of the secreted materials. Further, secretion is not equivalent to biosynthesis; most all (most or all?) tissues sequester ouabain from the circulation. When such tissues are removed from the circulation they secrete ouabain but this phenomenon, by itself, is not proof of biosynthesis.
The mode of action and significance of physiological levels of endogenous ouabain are under active investigation. In human plasma from healthy individuals, the circulating levels are normally distributed in the population and range typically from 30 - 380 pM. Significantly higher levels of endogenous ouabain that may approach or even exceed 1 nM have been observed in many patients with congestive heart failure, essential hypertension, renal failure and some cancers. It has been suggested that physiological concentrations of ouabain promote cell growth and in some manner stimulate the Na+/K+-ATPase activity while the higher levels achieved during intravenous therapy or in pathophysiological disorders may inhibit the Na+/K+-ATPase.[1]
In the years following the discovery of ouabain in the human circulation, it was found in the adrenal glands of cows[9] and dogs.[10]
Uses
Extracts containing ouabain have long been used by Somali tribesmen and other groups to poison hunting arrows.[11] A sufficiently concentrated ouabain dart can bring down a Hippopotamus probably as the result of respiratory and/or cardiac arrest.
In France and Germany, intravenous ouabain has a long history in the treatment of heart failure, and some continue to advocate its use intravenously and per os (orally) in angina pectoris and myocardial infarction. The positive properties of ouabain regarding the prophylaxis and treatment of these two indications are documented by a clutch of studies.[12] Ouabain isolated from plants is widely used by scientists in in vitro studies to specifically block the sodium pump (Na-K-ATPase). In many non-rodent species, low concentrations of this substance (i.e., in the subnanomolar range) may stimulate the Na-K-ATPase. The mechanism of the stimulatory effect is not understood and remains controversial. Further, the issue in rodents is more complicated because there are different isoforms of the Na-K-ATPase - some of which are very sensitive to ouabain while others are not.
The parenteral absorption seems to be better than indicated by the textbooks.[12]
Recently, use of ouabain as a contraceptive has been investigated, showing that it can severely decrease the motility of spermatozoa.[13]
References
- ↑ Gao J, Wymore RS, Wang Y, Gaudette GR, Krukenkamp IB, Cohen IS, Mathias RT (2002). "Isoform-Specific Stimulation of Cardiac Na/K Pumps by Nanomolar Concentrations of Glycosides" (pdf). J Gen Physiol. 119 (4): 297–312. doi:10.1085/jgp.20028501. PMC 2238186. PMID 11929882.
- ↑ Balzan S, d'Urso G, Nicolini G, Forini F, Pellegrino M, Montali U (2007). "Erythrocyte Sodium Pump Stimulation by Ouabain and an Endogenous Ouabain-Like Factor". Cell Biochem Funct. 25 (3): 297–303. doi:10.1002/cbf.1387. PMID 17191274.
- ↑ Aperia A (2007). "New Roles for an Old Enzyme: Na,K-ATPase Emerges as an Interesting Drug Target". J Intern Med. 261 (1): 44–52. doi:10.1111/j.1365-2796.2006.01745.x. PMID 17222167.
- ↑ Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH (1991). "Identification and Characterization of an Ouabain-Like Compound from Human Plasma". Proc Natl Acad Sci U S A. 88 (14): 6259–63. doi:10.1073/pnas.88.14.6259. PMC 52062. PMID 1648735.
- ↑ Hamlyn JM, Laredo J, Shah JR, Lu ZR, Hamilton BP (2003). "11-Hydroxylation in the Biosynthesis of Endogenous Ouabain: Multiple Implications". Ann N Y Acad Sci. 986: 685–93. doi:10.1111/j.1749-6632.2003.tb07283.x. PMID 12763919.
- ↑ Laredo J, Hamilton BP, Hamlyn JM (1994). "Ouabain is Secreted by Bovine Adrenocortical Cells". Endocrinology. 135 (2): 794–7. doi:10.1210/en.135.2.794. PMID 8033829.
- ↑ Murrell JR, Randall JD, Rosoff J, Zhao JL, Jensen RV, Gullans SR, Haupert GT Jr (2005). "Endogenous Ouabain: Upregulation of Steroidogenic Genes in Hypertensive Hypothalamus but not Adrenal" (pdf). Circulation. 112 (9): 1301–8. doi:10.1161/CIRCULATIONAHA.105.554071. PMID 16116051.
- ↑ d'Urso G, Frascarelli S, Balzan S, Zucchi R, Montali U (2004). "Production of Ouabain-Like Factor in Normal and Ischemic Rat Heart". J Cardiovasc Pharmacol. 43 (5): 657–62. PMID 15071352.
- ↑ Schneider R, Wray V, Nimtz M, Lehmann WD, Kirch U, Antolovic R, Schoner W (1998). "Bovine Adrenals Contain, in Addition to Ouabain, a Second Inhibitor of the Sodium Pump". The Journal of Biological Chemistry. 273 (2): 784–92. doi:10.1074/jbc.273.2.784. PMID 9422732.
- ↑ Boulanger BR, Lilly MP, Hamlyn JM, Laredo J, Shurtleff D, Gann DS (1993). "Ouabain is secreted by the adrenal gland in awake dogs". The American Journal of Physiology: Endocrinology and Metabolism. 264 (3): E413–E419. PMID 8460688.
- ↑ "On the Munchi arrow poison and strophanthin", J Physiol. May 6, 1908, George Ralph Mines
- ↑ 12.0 12.1 Fürstenwerth H (2010). "Ouabain - The Insulin of the Heart". Int J Clin Pract. 64 (12): 1591–4. doi:10.1111/j.1742-1241.2010.02395.x. PMID 20946265.
- ↑ Clausen MJ, Nissen P, Poulsen H (2011). "The Pumps that Fuel a Sperm's Journey". Biochem Soc Trans. 39 (3): 741–5. PMID 21599643.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Cardiac glycosides
- Cardiovascular Drugs
- Drug