Fospropofol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Fospropofol is a general anesthetic that is FDA approved for the {{{indicationType}}} of monitored anesthesia care sedation.. Common adverse reactions include cardiovascular: hypoxemia (1% to 27% ), dermatologic: pruritus (16% to 28% ), neurologic: paresthesia (49% to 74%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Monitored anesthesia care sedation: healthy adults or adults with mild systemic disease (American Society of Anesthesiology physical status of P1 or P2) 18 to 65 years of age, initiation, 6.5 mg/kg IV bolus followed immediately by supplemental infusion; patients weighing less than 60 kg should be dosed at 60 kg and patients weighing greater than 90 kg should be dosed at 90 kg.
- Monitored anesthesia care sedation: healthy adults or adults with mild systemic disease (American Society of Anesthesiology physical status of P1 or P2) 18 to 65 years of age, supplemental, 1.6 mg/kg IV no more frequently than every 4 min as needed to achieve the desired level of sedation; patients weighing less than 60 kg should be dosed at 60 kg and patients weighing greater than 90 kg should be dosed at 90 kg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Fospropofol in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Fospropofol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of fospropofol injection has not been established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Fospropofol in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Fospropofol in pediatric patients.
Contraindications
- None.
Warnings
Monitoring
- Fospropofol should be administered only by persons trained in the administration of general anesthesia and not involved in the conduct of the diagnostic or therapeutic procedure. Sedated patients should be continuously monitored, and facilities for maintenance of a patent airway, providing artificial ventilation, administering supplemental oxygen, and instituting cardiovascular resuscitation must be immediately available. Patients should be continuously monitored during sedation and through the recovery process for early signs of hypotension, apnea, airway obstruction, and/or oxygen desaturation.
Respiratory Depression
- Fospropofol may cause loss of spontaneous respiration. Apnea was reported in 1/455 (< 1%) patients treated with Fospropofol using the standard or modified dosing regimen [see Dosage and Administration]. In patients treated with greater than the recommended Fospropofol dose, apnea was reported in 14/556 (3%).
- Supplemental oxygen is recommended for all patients receiving Fospropofol. Dosages of Fospropofol must be individualized for each patient and titrated to effect [see Dosage and Administration (2.1) and Clinical Pharmacology (12.2)]. Use lower doses of Fospropofol in patients who are ≥65 years of age or who have severe systemic disease [see Dosage and Administration (2.3)]. The additive cardiorespiratory effects of narcotic analgesics and sedative-hypnotic agents should be considered when administered concomitantly with Fospropofol.
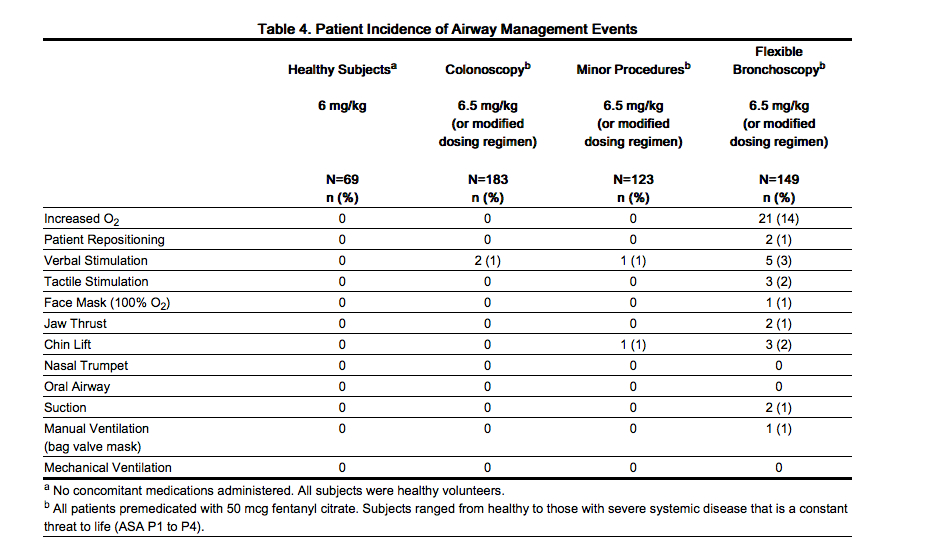
- Patients should be assessed for their ability to demonstrate purposeful response while sedated with Fospropofol as patients who are unable to do so may lose protective reflexes. Airway assistance maneuvers may be required in the management of respiratory depression (see Table 4).
Hypoxemia
- Fospropofol may cause hypoxemia detectable by pulse oximetry. Hypoxemia was reported in 20/455 (4%) patients treated with Fospropofol using the standard or modified dosing regimen [see Dosage and Administration (2.2, 2.3)]. Hypoxemia was reported among patients who retained the ability to respond purposefully to their health care provider following administration of Fospropofol. Therefore, retention of purposeful responsiveness did not prevent patients from becoming hypoxemic following administration of Fospropofol. In patients treated with greater than the recommended Fospropofol dose, hypoxemia was reported in 151/556 (27%).
- The risk of hypoxemia is reduced by appropriate positioning of the patient and the use of supplemental oxygen in all patients receiving Fospropofol. Airway assistance maneuvers may be required in the management of hypoxemia (see Table 4). The additive cardiorespiratory effects of narcotic analgesics and other sedative-hypnotic agents should be considered when administered concomitantly with Fospropofol.
Patient Unresponsiveness to Vigorous Tactile or Painful Stimulation
- Fospropofol has not been studied for use in general anesthesia. However, administration of Fospropofol may inadvertently cause patients to become unresponsive or minimally responsive to vigorous tactile or painful stimulation. The incidence of patients sedated for colonoscopy who became minimally responsive or unresponsive to vigorous tactile or painful stimulation was 7/183 (4%). The duration of minimal or complete unresponsiveness in colonoscopy patients ranged from 2 to 16 minutes. Among patients sedated for bronchoscopy, the incidence of patients who became minimally or completely unresponsive to vigorous tactile or painful stimulation was 24/149 (16%). The duration of minimal to complete unresponsiveness in bronchoscopy patients ranged from 2 to 20 minutes.
Hypotension
- Hypotension following the use of Fospropofol may occur. Hypotension was reported in 18/455 (4%) patients treated with Fospropofol using the standard or modified dosing regimen [see Dosage and Administration]. In patients treated with greater than the recommended Fospropofol dose, hypotension was reported in 31/556 (6%).
- Patients with compromised myocardial function, reduced vascular tone, or who have reduced intravascular volume may be at an increased risk for hypotension. A secure intravenous access catheter and supplemental volume replacement fluids should be readily available during the procedure. Additional pharmacological management may be necessary.
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions are discussed elsewhere in the labeling:
- Respiratory depression [see Warnings and Precautions]
- Hypoxemia [see Warnings and Precautions]
- Loss of purposeful responsiveness [see Warnings and Precautions]
- Hypotension [see Warnings and Precautions]
- The most common adverse reactions (reported in greater than 20%) are paresthesia and pruritus.
- The most commonly reported reasons for discontinuation are paresthesia and cough.
Clinical Trials Experience
- Adverse reactions presented in this section are derived from 332 patients in 3 controlled clinical trials in patients undergoing colonoscopy or flexible bronchoscopy and 123 patients in one open-label study in patients undergoing minor procedures. Patients enrolled in the studies who received the standard or modified dosing regimen included males and females, ≥18 years of age and ranging from healthy (359/455 [79%] ASA P1 or P2) to those with severe systemic disease (96/455 [21%] ASA P3 or P4). Of the 455 patients enrolled, 345 (76%) were ≥18 to <65 years of age and 110 (24%) were ≥65 years of age. Adverse reactions are reported for patients who received the standard or the modified dosing regimen [see Dosage and Administration]. The majority of procedures were less than thirty minutes in duration. All patients in these studies received 50 mcg fentanyl citrate intravenously as premedication, and some of the patients received additional 25 mcg fentanyl citrate supplemental doses. Adverse reactions occurring in ≥2% of patients in these studies are presented in Table 3.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not accurately reflect the rates observed in practice.
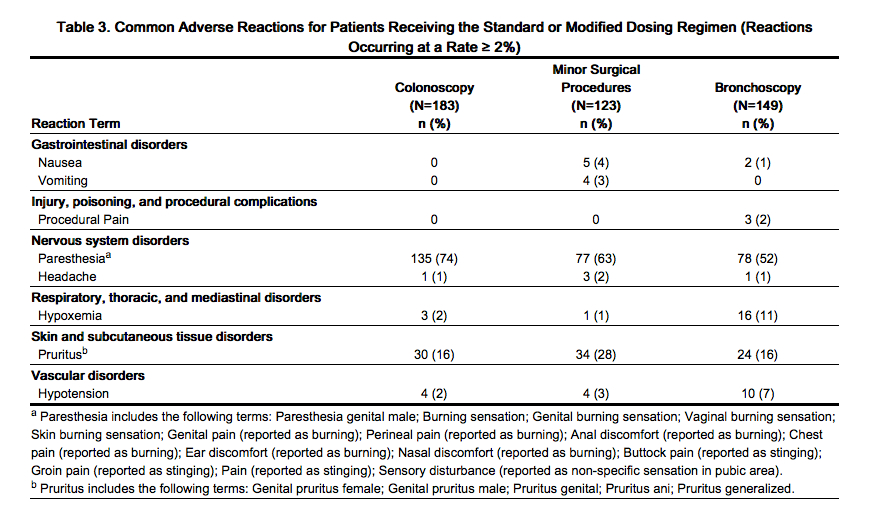
- Paresthesias (including burning, tingling, stinging) and/or pruritus, usually manifested in the perineal region, were the most frequently recorded adverse reactions in clinical trials. Paresthesias and pruritus generally occurred within 5 minutes after administration of the initial dose of Fospropofol and were generally transient and mild to moderate in intensity. The pharmacologic basis of these sensory phenomena is unknown. No pretreatments, including the use of nonsteroidal anti-inflammatory drugs, opioids, or lidocaine, are known to have an effect on or to reduce the incidence of these sensations.
- Sedation-related adverse reactions were experienced at the following rates for subjects receiving the standard or modified Fospropofol dosing regimen: 20/455 (4%) hypoxemia, 18/455 (4%) hypotension, 1/455 (< 1%) apnea. A greater rate of sedation-related adverse reactions necessitating intervention was observed in patients undergoing bronchoscopy compared with colonoscopy and minor surgical procedures. In the colonoscopy studies, 5/183 (3%) patients were ASA P3. In the minor surgical procedures study, 23/123 (19%) patients were ASA P3 or P4. In the flexible bronchoscopy study, 68/150 (46%) patients were ASA P3 or P4. The type and incidence of airway assistance interventions required for patients who experienced sedation-related adverse reactions are presented in Table 4.
Adverse Reactions in Prolonged Exposure in Adults
- The safety of Fospropofol for continuous sedation has not been established and therefore its use is not recommended. Fospropofol was administered to 38 intubated and mechanically ventilated patients in postoperative and intensive care settings. An occurrence of nonsustained ventricular tachycardia was observed as a serious adverse reaction in one patient in the study. Another patient with acute myeloid leukemia with renal and hepatic insufficiency experienced a further increase in plasma formate concentration from a baseline of 66 mcg/mL to a post-dose level of 212 mcg/mL after a 12-hour infusion. The clinical significance of these findings is unknown.
Postmarketing Experience
There is limited information regarding Fospropofol Postmarketing Experience in the drug label.
Drug Interactions
- Fospropofol may produce additive cardiorespiratory effects when administered with other cardiorespiratory depressants such as sedative-hypnotics and narcotic analgesics.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- Pregnancy Category B.
- There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Reproduction studies have been performed in rats and rabbits at doses up to 0.6 and 1.7 times the anticipated human dose for a procedure of 16 minutes based on a comparison of doses expressed as mg/m2 and have revealed no evidence of impaired fertility or harm to the fetus due to Fospropofol.
- Pregnant rats were treated with fospropofol disodium (5, 20, or 45 mg/kg/day, IV) from gestation day 7 through 17 (the highest dose is 0.6 times the anticipated human dose for a procedure of 16 minutes based on a comparison of doses expressed as mg/m2). Doses of 20 and 45 mg/kg/day produced significant maternal toxicity. No drug-related adverse effects on embryo-fetal development were noted.
- Pregnant rabbits were treated with fospropofol disodium (14, 28, 56 or 70 mg/kg/day, IV) from gestation day 6 through 18 (the highest dose is 1.7 times the anticipated human dose for a procedure of 16 minutes based on a comparison of doses expressed as mg/m2). Significant maternal toxicity was noted at all doses. No drug-related adverse effects on embryo-fetal development were noted.
Nonteratogenic Effects.
- Pregnant rats were administered 0, 5, 10, or 20 mg/kg/day fospropofol disodium from gestation day 7 through lactation day 20 to evaluate perinatal and postnatal development (the highest dose is 0.2 times the anticipated human dose for a procedure of 16 minutes based on a comparison of doses expressed as mg/m2). There were no clear treatment-related effects on growth, development, behavior (passive avoidance and water maze) or fertility and mating capacity of the offspring.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fospropofol in women who are pregnant.
Labor and Delivery
- Fospropofol is not recommended for use in labor and delivery, including Cesarean section deliveries. It is not known if fospropofol crosses the placenta; however, propofol is known to cross the placenta, and as with other sedative-hypnotic agents, the administration of Fospropofol may be associated with neonatal respiratory and cardiovascular depression.
Nursing Mothers
- It is not known whether fospropofol is excreted in human milk; however, propofol has been reported to be excreted in human milk, and the effects of oral absorption of fospropofol or propofol are not known. Fospropofol is not recommended for use in nursing mothers.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established because Fospropofol has not been studied in persons <18 years of age. Fospropofol is not recommended for use in this population.
Geriatic Use
- In studies of Fospropofol for sedation in brief diagnostic and therapeutic procedures, 17% of patients were ≥65 years of age and 5% of patients were ≥75 years of age. Patients ≥65 years of age should receive the modified dosing regimen [see Dosage and Administration (2.3)]. Hypoxemia was reported more frequently among patients aged ≥75 years than among patients aged 65 to <75 years and less frequently among younger patients, aged 18 to < 65 years.
Gender
There is no FDA guidance on the use of Fospropofol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fospropofol with respect to specific racial populations.
Renal Impairment
- In studies of Fospropofol for sedation in brief diagnostic and therapeutic procedures, 21% of patients had a creatinine clearance <80 mL/min, and 4% had a creatinine clearance <50 mL/min. Pharmacokinetics of fospropofol or propofol were not altered in patients with mild to moderate renal insufficiency. No dosing adjustments are required for patients with creatinine clearance ≥30 mL/min. Limited safety and efficacy data are available for Fospropofol in patients with creatinine clearance < 30 mL/min.
Hepatic Impairment
- Fospropofol has not been adequately studied in patients with hepatic impairment. Caution should be exercised when using fospropofol disodium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fospropofol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fospropofol in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Fospropofol Administration in the drug label.
Monitoring
There is limited information regarding Fospropofol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Fospropofol and IV administrations.
Overdosage
- Overdosage with Fospropofol can cause cardiorespiratory depression. If overdosage occurs, Fospropofol administration should be discontinued immediately. Respiratory depression may require manual or mechanical ventilation. Cardiovascular depression may require elevation of lower extremities, intravascular volume replacement, and/or pharmacological management.
- Formate and phosphate are metabolites of Fospropofol and may contribute to signs of toxicity following overdosage. Signs of formate toxicity are similar to those of methanol toxicity and are associated with anion-gap metabolic acidosis. Intravenous exposure to a large amount of phosphate could potentially cause hypocalcemia with paresthesia, muscle spasms, and seizures.
Pharmacology
| |
Fospropofol
| |
| Systematic (IUPAC) name | |
| disodium [2,6-di(propan-2-yl)phenoxy]methyl phosphate[1] | |
| Identifiers | |
| CAS number | |
| ATC code | N01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 332.240261 g/mol[1] |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 98%[2] |
| Metabolism | Hepatic glucuronidation |
| Half life | 0.81 hours[2] |
| Excretion | Renal |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
B |
| Legal status | |
| Dependence Liability | unknown |
| Routes | Intravenous |
Mechanism of Action
- Fospropofol disodium is a prodrug of propofol. Following intravenous injection, fospropofol is metabolized by alkaline phosphatases. For every millimole of fospropofol disodium administered, one millimole of propofol is produced (1.86 mg of fospropofol disodium is the molar equivalent of 1 mg propofol).
Structure
- Fospropofol is an injection solution intended for intravenous administration as a sedative-hypnotic agent. Fospropofol is an aqueous, sterile, nonpyrogenic, clear, colorless, iso-osmotic solution containing 35 mg/mL of fospropofol disodium. Fospropofol disodium is a water-soluble prodrug of propofol, chemically described as 2,6-diisopropylphenoxymethyl phosphate, disodium salt. The structural and molecular formulas are shown in Figure 1.
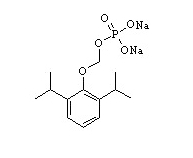
- Molecular Formula: C13H19O5PNa2
- Molecular Weight: 332.24
- Figure 1. Structural and Molecular Formulas of Fospropofol Disodium
- The inactive components include monothioglycerol (0.25 wt%) and tromethamine (0.12 wt%). LUSEDRA has a pH of 8.2 to 9.0. LUSEDRA does not contain any antimicrobial preservatives and is intended for single-use administration.
Pharmacodynamics
- The pharmacology of fospropofol, once metabolized to propofol, is comparable to that of propofol lipid emulsion; however, the liberation of propofol from fospropofol results in differences in the timing of the pharmacodynamic effects. To characterize the pharmacokinetic/pharmacodynamic (PK/PD) profile of propofol derived from Fospropofol, 12 healthy subjects were administered a 10-mg/kg intravenous bolus dose of Fospropofol, and the sedative effect was measured as a decrease in Modified Observer's Assessment of Alertness/Sedation (MOAA/S) score (Table 5).2 The PK and PD results are shown in Figure 2. Peak plasma levels of propofol (2.2 ± 0.4 μg/mL) released from fospropofol were noted by 8 minutes (range 4 - 13 minutes) and minimum mean MOAA/S score of 1.2 (range 0 - 3) was noted in 7 minutes (range 1 - 15 minutes). Subjects completely recovered from sedative effects between 21 to 45 minutes after Fospropofol administration.
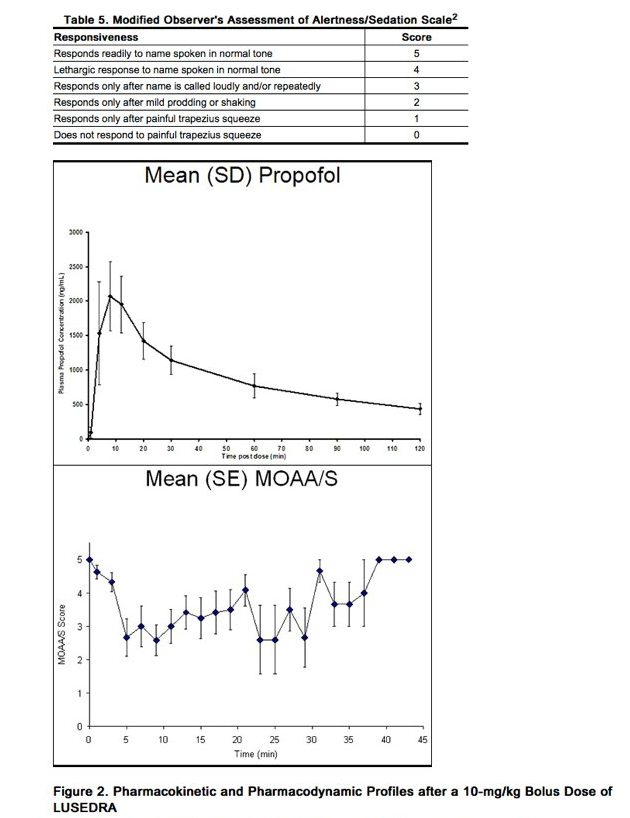
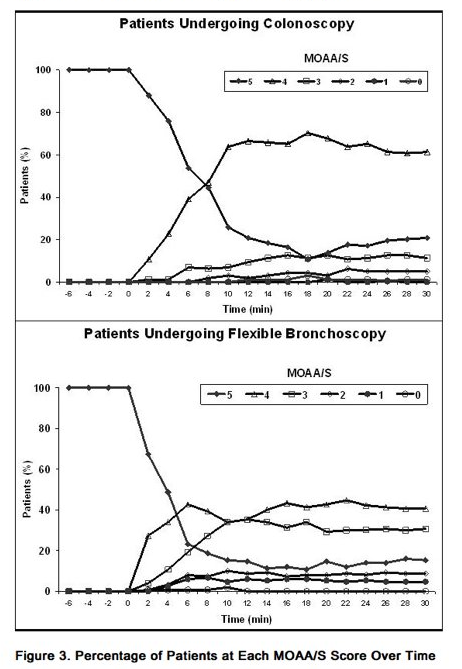
- Fospropofol was evaluated in randomized, blinded, dose-controlled studies for sedation in patients undergoing colonoscopy and flexible bronchoscopy [see Clinical Studies (14.1)]. Figure 3 shows MOAA/S scores over time in each of the studies for those patients who received the standard and modified dosing regimens. In the study of patients undergoing colonoscopy, patients who received the standard and modified dosing regimens had a median [range] time to sedation (time from first dose of sedative to the first of 2 consecutive MOAA/S scores of ≤ 4) of 8.0 [2, 28] minutes and a median time to Fully Alert (3 consecutive responses to their name spoken in a normal tone, measured every 2 minutes beginning at or after the end of the procedure) of 5.0 [0, 47] minutes. In the study of patients undergoing flexible bronchoscopy, patients who received the standard and modified Fospropofol dosing regimens had a median time to sedation of 4 [2, 22] minutes and a median time to Fully Alert of 5.5 [0, 61] minutes.
Fospropofol-04
- Within the recommended dose range, there were no differences in matched QTc interval changes between Fospropofol and placebo. The effect of Fospropofol on the QTcF interval was measured in a crossover study in which healthy subjects (n=68) received the following treatments: 6-mg/kg intravenous Fospropofol; 18-mg/kg intravenous Fospropofol; moxifloxacin 400 mg orally (positive control); and normal saline IV. After baseline and placebo adjustment, the maximum mean QTcF change was 2 ms (1-sided 95% Upper CI: 6 ms) for the 6-mg/kg dose and 8 ms (1-sided 95% Upper CI: 12 ms) for the 18-mg/kg dose. Used as a positive control, moxifloxacin had a maximum mean change in QTcF of 12 ms (1-sided 95% Lower CI: 6 ms).
Pharmacokinetics
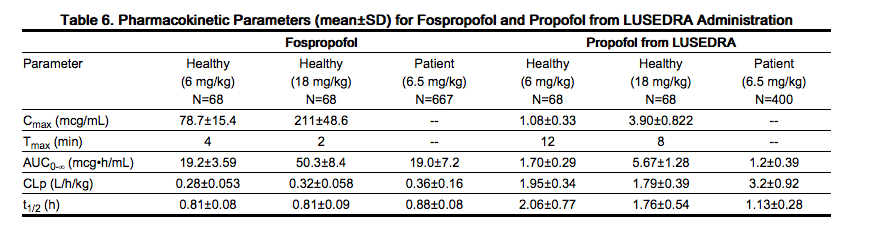
PK parameters were evaluated in a crossover study of 68 healthy subjects, 18 to 45 years of age, who received 6- and 18-mg/kg intravenous bolus doses of Fospropofol. PK parameters are shown in Table 6. The Cmax and AUC0-∞ values of fospropofol were dose proportional. The intersubject variability in Cmax and AUC0-∞ was low. Propofol was rapidly liberated reaching plasma Cmax at a median Tmax of 12 minutes for Fospropofol 6 mg/kg and 8 minutes for Fospropofol 18 mg/kg. Concentration-time profiles showed a biexponential decline. The increase in Cmax and AUC0-∞ of propofol was dose proportional.
Distribution
- Fospropofol has a low volume of distribution of 0.33±0.069 L/kg, and the liberated propofol has a large volume of distribution (5.8 L/kg).
- Both fospropofol and its active metabolite propofol are highly protein bound (approximately 98%), primarily to albumin. Fospropofol does not affect the binding of propofol to albumin.
Metabolism
- Fospropofol is completely metabolized by alkaline phosphatases to propofol, formaldehyde, and phosphate. Formaldehyde and phosphate plasma concentrations are comparable to endogenous levels when fospropofol disodium is administered as recommended. Formaldehyde is further metabolized to formate by several enzyme systems, including formaldehyde dehydrogenase, present in various tissues. Propofol liberated from fospropofol is further metabolized to major metabolites propofol glucuronide (34.8%), quinol-4-sulfate (4.6%), quinol-1-glucuronide (11.1%), and quinol-4-glucuronide (5.1%). Oxidation to CO2 is the primary means of eliminating excess formate.
- Fospropofol is not a substrate of CYP450 enzymes.
Elimination
- After a single 400 mg intravenous dose of [14C]-fospropofol disodium in humans, approximately 71% of radioactivity was recovered in the urine within 192 hours. Total body clearance (CLp) of fospropofol was 0.280±0.053 L/h/kg, and renal elimination of fospropofol was insignificant (<0.02% of dose). The terminal phase elimination half-life (t1/2) of fospropofol was 0.81±0.08 and 0.88±0.08 hours in healthy subjects and patients, respectively. In healthy subjects, the apparent total body clearance of liberated propofol (CLp/F) was 1.95±0.345 L/h/kg and t1/2 was 2.06±0.77 hours. In patients, the CLp of fospropofol was 0.31±0.14 L/h/kg and CLp/F for propofol was 2.74±0.80 L/h/kg and is similar to that observed in healthy subjects.
Special Populations
- Population pharmacokinetic analysis indicated no influence of race, gender, age, renal impairment or alkaline phosphatase concentrations on the pharmacokinetics of fospropofol. Pharmacokinetics of propofol derived from fospropofol was not influenced by race, gender, or renal impairment.
- Fospropofol has not been adequately studied in patients with hepatic impairment. Caution should be exercised when using fospropofol disodium in patients with hepatic impairment.
Drug Interactions
- There was no effect of analgesic premedication [fentanyl (1 mcg/kg); meperidine (0.75 mg/kg); midazolam (0.01 mg/kg); morphine (0.1 mg/kg)] on plasma pharmacokinetics of fospropofol.
- In an in vitro protein-binding study, there was no significant interaction between fospropofol and propofol at concentrations up to 200 mcg/mL and 5 mcg/mL, respectively. The interaction of fospropofol with other highly protein-bound drugs given concomitantly has not been studied.
- Potential of fospropofol or its major metabolite, propofol, to inhibit or induce major cytochrome P450 enzymes is not known.
Nonclinical Toxicology
Carcinogenesis
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of fospropofol disodium.
Mutagenesis
- Fospropofol was not genotoxic in the Ames bacterial reverse mutation assay, with or without metabolic activation, and in the in vivo mouse micronucleus assay. Fospropofol was positive in the L5178Y TK+/- mouse lymphoma forward mutation assay in the presence of metabolic activation. In contrast, fospropofol was negative in this assay in the presence of formaldehyde-metabolizing enzymes suggesting that the positive finding is likely due to an artifact of the culture conditions.
Impairment of Fertility
- Male rats were treated with 5, 10, or 20 mg/kg fospropofol for 4 weeks prior to mating. Male fertility was not altered in animals treated with 20 mg/kg (0.3-fold the total human dose for a procedure of 16 minutes based on a mg/m2 basis).
- Female rats were treated with 5, 10, or 20 mg/kg fospropofol for two weeks prior to mating. There were no clear treatment-related effects on female fertility at a dose of 20 mg/kg (0.3-fold the total human dose for a procedure of 16 minutes based on a mg/m2 basis).
Clinical Studies
Use in Sedation for Diagnostic or Therapeutic Procedures
- The standard and modified Fospropofol dosing regimens were evaluated in two controlled studies in patients dosed with Fospropofol who were over 18 years of age and undergoing diagnostic or therapeutic procedures. All patients received 50 mcg of fentanyl citrate intravenously before study sedative medication. The primary endpoint was the rate of "sedation success," defined as the proportion of patients who did not respond readily to their name spoken in a normal tone of voice (Modified Observer's Assessment of Alertness/Sedation Scale score of 4 or less) on 3 consecutive measurements taken every 2 minutes and who completed the procedure without the use of alternative sedative medication and without the use of manual or mechanical ventilation. 2
- In both studies, an initial bolus dose and up to 3 supplemental doses at 25 % of the initial bolus of study sedative medication were administered intravenously to sedate patients so that they did not respond readily to their name spoken in a normal tone and to allow the investigator to start the procedure. During the procedure, supplemental doses at 25% of the initial bolus were allowed to maintain sedation. Patients who were not adequately sedated with study drug received alternative sedative medication per the site's standard of care; however, sites were instructed not to use propofol as it would interfere with PK measurements.
- The standard and modified Fospropofol dosing regimens were evaluated in a randomized, blinded, dose-controlled study for sedation in patients undergoing colonoscopy. All of the patients who received alternative sedative medication (n=19) received midazolam. Patients randomized to receive the Fospropofol standard or modified dosing regimen had a sedation success rate of 87% and required a mean number of supplemental doses of 2.3 (±1.4 SD). Patients randomized to receive Fospropofol had a median procedure duration of 11 minutes.
- The standard and modified Fospropofol dosing regimens were also evaluated in a randomized, blinded, dose-controlled study for sedation in patients undergoing flexible bronchoscopy. All of the patients who received alternative sedative medication (n=12) received midazolam. Patients randomized to receive the Fospropofol standard or modified dosing regimen had a sedation success rate of 89% and required a mean number of supplemental doses of 1.7 (±1.6 SD). Patients randomized to Fospropofol had a median procedure duration of 10 minutes.
How Supplied
- Fospropofol, 35 mg/mL (total of 1,050 mg/30 mL) fospropofol disodium, is supplied as a single-use, aqueous, sterile, nonpyrogenic, clear, colorless solution in glass vials ready for intravenous injection. Each vial is filled with 32.1 mL intended to deliver a minimum of 30 mL of fospropofol disodium solution.
Storage
- Store at controlled room temperature 25°C (77°F). Excursions permitted between 15° and 30°C (59° and 86°F).
- NDC 62856-350-08.
Images
Drug Images
{{#ask: Page Name::Fospropofol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fospropofol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Paresthesias (including burning, tingling, stinging) and/or pruritus, usually manifested in the perineal region are frequently experienced upon injection of the initial dose of Fospropofol. Inform the patient that these sensations are typically mild to moderate in intensity, last a short time, and require no treatment.
- Requirement for a patient escort should be considered. The decision as to when patients who have received Fospropofol, particularly on an outpatient basis, may again engage in activities requiring complete mental alertness, coordination and/or physical dexterity (e.g., operate hazardous machinery, sign legal documents, or drive a motor vehicle) must be individualized.
- Eisai Inc.
- 100 Tice Boulevard
- Woodcliff Lake, NJ 07677
- USA
- LUSEDRA is a trademark used by Eisai Inc. under license from Eisai R&D Management Co., Ltd.
- ©2009 Eisai Inc.
- All rights reserved. 10/09
Precautions with Alcohol
Alcohol-Fospropofol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Fospropofol Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Fospropofol Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 PubChem Compound. = pccompound&term = fospropofol "fospropofol disodium - Compound Summary (CID 3038497)" Check
|url=value (help). Bethesda, Maryland: National Center for Biotechnology Information. Retrieved 2 August 2010. External link in|publisher=(help) - ↑ 2.0 2.1 Eisai Inc. (October 2009). "LUSEDRA (fospropofol disodium) Injection" (PDF). Woodcliff Lake, New Jersey: Eisai Inc. Retrieved 2 August 2010. External link in
|publisher=(help)
{{#subobject:
|Label Page=Fospropofol |Label Name=Fospropofol label.png
}}