Fluorescein (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Fluorescein (injection) is a contrast media that is FDA approved for the diagnosis of fluorescein angiography or angioscopy of the retina and iris vasculature. Common adverse reactions include hypersensitivity.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Fluorescein injection, USP 10% is indicated in diagnostic fluorescein angiography or angioscopy of the retina and iris vasculature.
Dosage
- The normal adult dose of fluorescein injection, USP 10% is 500 mg (100 mg/mL) via intravenous administration.
- For children, the dose should be calculated on the basis of 35 mg for each ten pounds of body weight (7.7 mg/kg body weight).
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not mix or dilute with other solutions or drugs. Flush intravenous cannulas before and after drugs are injected to avoid physical incompatibility reactions.
- Inject the dose rapidly (1 mL per second is normally recommended) into the antecubital vein, after taking precautions to avoid extravasation. A syringe, filled with fluorescein injection, USP 10%, may be attached to transparent tubing and a 23 gauge butterfly needle for injection. Insert the needle and draw the patient's blood to the hub of the syringe so that a small air bubble separates the patient's blood in the tubing from the fluorescein. With the room lights on, slowly inject the blood back into the vein while watching the skin over the needle tip. If the needle has extravasated, the patient's blood will be seen to bulge the skin and the injection should be stopped before any fluorescein is injected. When assured that extravasation has not occurred, the room light may be turned off and the fluorescein injection completed. Luminescence usually appears in the retina and choroidal vessels in 7 to 14 seconds and can be observed by standard viewing equipment.
- Reduction in dose from 5 ml to 2 ml of fluorescein injection, USP 10% may be appropriate in cases when a highly sensitive imaging system e.g., scanning laser ophthalmoscope is used.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fluorescein (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fluorescein (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Fluorescein (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Fluorescein (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Fluorescein (injection) in pediatric patients.
Contraindications
- FLUORESCITE® (fluorescein injection, USP) 10% is contraindicated in patients with known hypersensitivity to fluorescein sodium or any other ingredients in this product.
Warnings
FOR INTRAVENOUS USE
- Care must be taken to avoid extravasation during injection as the high pH of fluorescein solution can result in severe local tissue damage. The following complications resulting from extravasation of fluorescein have been noted to occur: Sloughing of the skin, superficial phlebitis, subcutaneous granuloma, and toxic neuritis along the median curve in the antecubital area. Complications resulting from extravasation can cause severe pain in the arm for up to several hours. When significant extravasation occurs, the injection should be discontinued and conservative measures to treat damaged tissue and to relieve pain should be implemented. Rare cases of death due to anaphylaxis have been reported.
Adverse Reactions
Clinical Trials Experience
- Nausea, vomiting, gastrointestinal distress, headache, syncope, hypotension, and symptoms and signs of hypersensitivity have occurred. Cardiac arrest, basilar artery ischemia, severe shock, convulsions, thrombophlebitis at the injection site, and rare cases of death have been reported.
- Extravasation of the solution at the injection site causes intense pain at the site and a dull aching pain in the injected arm. Generalized hives and itching, bronchospasm and anaphylaxis have been reported. A strong taste may develop after injection.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Fluorescein (injection) in the drug label.
Drug Interactions
There is limited information regarding Fluorescein (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Adequate animal reproduction studies have not been conducted with fluorescein sodium. It is also not known whether fluorescein sodium can cause fetal harm when administered to a pregnant woman. Fluorescein sodium should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fluorescein (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Fluorescein (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Fluorescein (injection) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Fluorescein (injection) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Fluorescein (injection) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Fluorescein (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fluorescein (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Fluorescein (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Fluorescein (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fluorescein (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fluorescein (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Fluorescein (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Fluorescein (injection) in the drug label.
Overdosage
There is limited information regarding Fluorescein (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
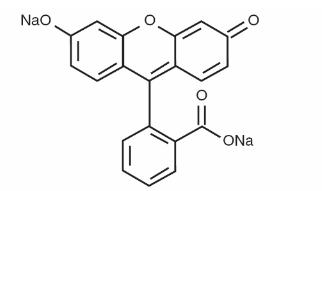
- Fluorescein sodium responds to electromagnetic radiation and light between the wavelengths of 465-490 nm and fluoresces, i.e., emits light at wavelengths of 520-530 nm. Thus, the hydrocarbon is excited by blue light and emits light that appears yellowish-green. Following intravenous injection of fluorescein sodium in an aqueous solution, the unbound fraction of the fluorescein can be excited with a blue light flash from a fundus camera as it circulates through the ocular vasculature, and the yellowish green fluorescence of the dye is captured by the camera. In the fundus, the fluorescence of the dye demarcates the retinal and/or choroidal vasculature under observation, distinguishing it from adjacent areas/structures.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Fluorescein (injection) in the drug label.
Pharmacokinetics
Distribution
- Within 7 to 14 seconds after IV administration into antecubital vein, fluorescein usually appears in the central artery of the eye. Within a few minutes of IV administration of fluorescein sodium, a yellowish discoloration of the skin occurs, which begins to fade after 6 to 12 hours of dosing. Various estimates of volume of distribution indicate that fluorescein distributes well into interstitial space (0.5 L/kg).
Metabolism
- Fluorescein undergoes rapid metabolism to fluorescein monoglucuronide. After IV administration of fluorescein sodium (14 mg/kg) to 7 healthy subjects, approximately 80% of fluorescein in plasma was converted to glucuronide conjugate after a period of 1 hour post dose, indicating relatively rapid conjugation.
Excretion
- Fluorescein and its metabolites are mainly eliminated via renal excretion. After IV administration, the urine remains slightly fluorescent for 24 to 36 hours. A renal clearance of 1.75 mL/min/kg and a hepatic clearance (due to conjugation) of 1.50 mL/min/kg have been estimated. The systemic clearance of fluorescein was essentially complete by 48 to 72 hours after administration of 500 mg fluorescein.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Fluorescein (injection) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Fluorescein (injection) in the drug label.
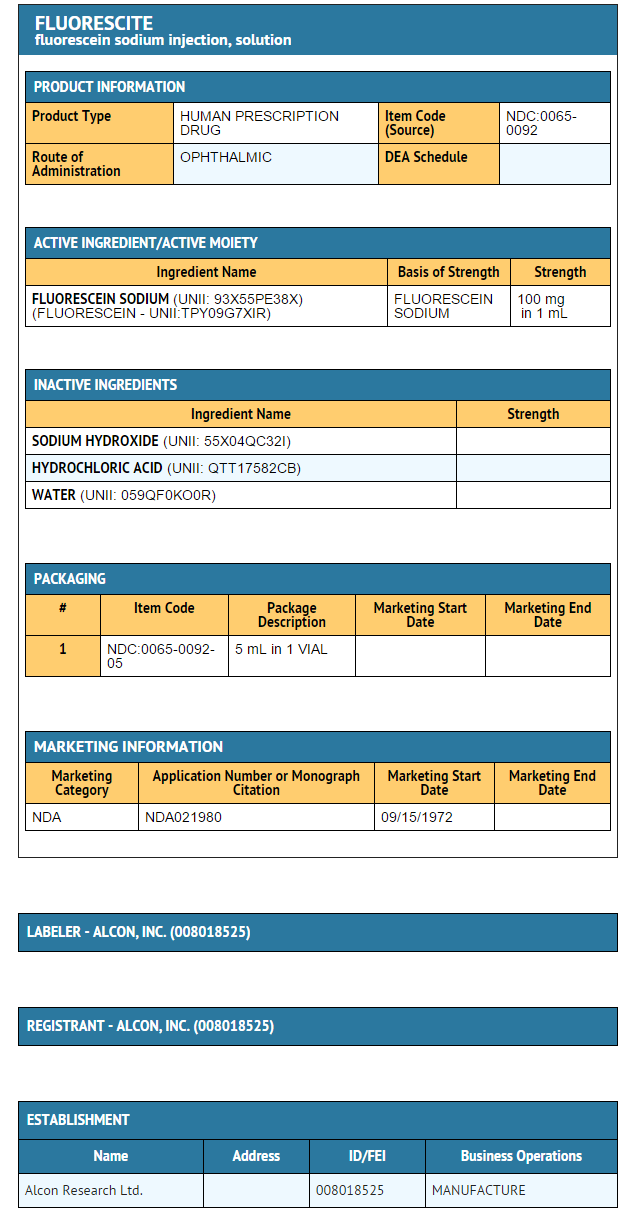
How Supplied
- FLUORESCITE® (fluorescein injection, USP) 10% is supplied in a single-use 5 mL glass vial with a gray FluroTec coated chlorobutyl (latex free) stopper and purple flip-off aluminum seal. It contains a sterile, red-orange solution of fluorescein sodium.
- NDC 0065-0092-65
Storage
- Store at 2°- 25°C (36°- 77°F).
- Do Not Freeze
Images
Drug Images
{{#ask: Page Name::Fluorescein (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Fluorescein (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Fluorescein (injection) in the drug label.
Precautions with Alcohol
- Alcohol-Fluorescein (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- FLUORESCITE®[1]
Look-Alike Drug Names
There is limited information regarding Fluorescein (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.