Florbetapir F-18
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Florbetapir F-18 is a Diagnostic Agent that is FDA approved for the diagnosis of Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD). Common adverse reactions include Hypertension, headache, Myalgia, Nausea, Injection site reaction.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Amyvid is indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD) and other causes of cognitive decline. A negative Amyvid scan indicates sparse to no neuritic plaques and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Amyvid scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition. Amyvid is an adjunct to other diagnostic evaluations.
Limitations of Use
- A positive Amyvid scan does not establish a diagnosis of AD or other cognitive disorder.
- Safety and effectiveness of Amyvid have not been established for:
- Predicting development of dementia or other neurologic condition;
- Monitoring responses to therapies.
Dosage
- Radiation Safety - Drug Handling
- Amyvid is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure during administration. Use waterproof gloves and effective shielding, including syringe shields when handling Amyvid. Radiopharmaceuticals, including Amyvid, should only be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radioactive materials, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radiopharmaceuticals.
Recommended Dosing and Administration Instructions
- The recommended dose for Amyvid is 370 MBq (10 mCi), maximum 50 μg mass dose, administered as a single intravenous bolus in a total volume of 10 mL or less. Follow the injection with an intravenous flush of 0.9% sterile sodium chloride.
- Inspect the radiopharmaceutical dose solution prior to administration and do not use it if it contains particulate matter or is discolored.
- Use aseptic technique and radiation shielding to withdraw Amyvid solution.
- Assay the dose in a suitable dose calibrator prior to administration.
- Inject Amyvid through a short intravenous catheter (approximately 1.5 inches or less) to minimize the potential for adsorption of the drug to the catheter. * Portions of the Amyvid dose may adhere to longer catheters.
Image Acquisition Guidelines
- A 10-minute PET image should be acquired starting 30 to 50 minutes after Amyvid intravenous injection. The patient should be supine and the head positioned to center the brain, including the cerebellum, in the PET scanner field of view. Reducing head movement with tape or other flexible head restraints may be employed. Image reconstruction should include attenuation correction with resulting transaxial pixel sizes between 2 and 3 mm.
Image Display and Interpretation
- Amyvid images should be interpreted only by readers who successfully complete a special training program. Training is provided by the manufacturer using either an in-person tutorial or an electronic process.
- The objective of Amyvid image interpretation is to provide an estimate of the brain β-amyloid neuritic plaque density, not to make a clinical diagnosis. Image interpretation is performed independently of a patient's clinical features and relies upon the recognition of unique image features.
Image Display
- Images should be displayed in the transaxial orientation with access as needed to the sagittal and coronal planes. In reviewing the images, include all transaxial slices of the brain using a black-white scale with the maximum intensity of the scale set to the maximum intensity of all the brain pixels. Initially locate the brain slice with the highest levels of image contrast (highest radioactivity signals for Amyvid uptake) and adjust the contrast appropriately. Start image interpretation by displaying slices sequentially from the bottom of the brain to the top. Periodically refer to the sagittal and coronal plane image display, as needed to better define the radioactivity uptake and to ensure that the entire brain is displayed.
Image Interpretation
- Image interpretation is based upon the distribution of radioactive signal within the brain; clinical information is not a component of the image assessment. Images are designated as positive or negative by comparing the radioactivity in cortical gray matter with activity in the adjacent white matter. This determination is made only in the cerebral cortex; the signal uptake in the cerebellum does not contribute to the scan interpretation (for example, a positive scan may show retained cerebellar gray-white contrast even when the cortical gray-white contrast is lost).
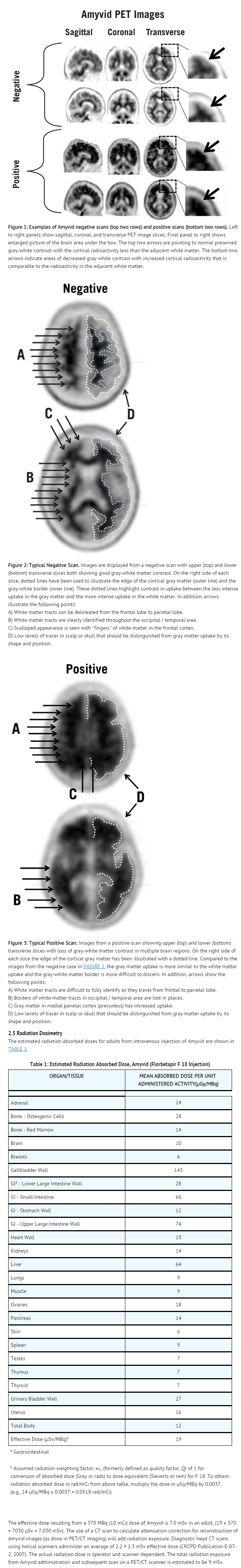
- Negative scans show more radioactivity in white matter than in gray matter, creating clear gray-white contrast.
Positive scans show cortical areas with reduction or loss of the normally distinct gray-white contrast. These scans have one or more areas with increased cortical gray matter signal which results in reduced (or absent) gray-white contrast. Specifically, a positive scan will have either:
- Two or more brain areas (each larger than a single cortical gyrus) in which there is reduced or absent gray-white contrast. This is the most common appearance of a positive scan.
or
- One or more areas in which gray matter radioactivity is intense and clearly exceeds radioactivity in adjacent white matter.
- Some scans may be difficult to interpret due to image noise, atrophy with a thinned cortical ribbon, or image blur. For cases in which there is uncertainty as to the location or edge of gray matter on the PET scan and a co-registered computerized tomography (CT) image is available (as when the study is done on a PET/CT scanner) the interpreter should examine the CT image to clarify the relationship of the PET radioactivity and the gray matter anatomy.
- FIGURES 1, 2, and 3 provide examples of negative and positive scans. FIGURE 1 demonstrates varying degrees of normal gray-white contrast (negative) and examples where gray-white contrast has been lost (positive). FIGURE 2 illustrates typical features of a negative scan, while FIGURE 3 shows the loss of gray-white contrast in different brain regions of a positive scan.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Florbetapir F-18 in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Florbetapir F-18 in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and efficacy in pediatric patients has not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Florbetapir F-18 in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Florbetapir F-18 in pediatric patients.
Contraindications
- None
Warnings
Risk for Image Misinterpretation and other Errors
- Errors may occur in the Amyvid estimation of brain neuritic plaque density during image interpretation.
- Image interpretation should be performed independently of the patient's clinical information. The use of clinical information in the interpretation of Amyvid images has not been evaluated and may lead to errors. Other errors may be due to extensive brain atrophy that limits the ability to distinguish gray and white matter on the Amyvid scan as well as motion artifacts that distort the image.
- Amyvid scan results are indicative of the brain neuritic amyloid plaque content only at the time of image acquisition and a negative scan result does not preclude the development of brain amyloid in the future.
Radiation Risk
- Amyvid, similar to other radiopharmaceuticals, contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk of cancer. Ensure safe handling to protect patients and health care workers from unintentional radiation exposure
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- In clinical studies, 555 patients were exposed to Amyvid. Amyvid caused no serious adverse reactions in the studies and the reported adverse reactions were predominantly mild to moderate in severity. The adverse reactions reported in more than one subject within the studies are shown in TABLE 2.
- Other adverse reactions occurred at lower frequencies and included infusion site rash, dysgeusia, pruritis, urticaria, and flushing.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Florbetapir F-18 in the drug label.
Drug Interactions
- Pharmacodynamic drug-drug interaction studies have not been performed in patients to establish the extent, if any, to which concomitant medications may alter Amyvid image results.
- Within a clinical study of patients with a range of cognitive impairment, some patients with probable AD were receiving the following medications: donepezil, galantamine, memantine. Mean cortical Standardized Uptake Value (SUV) ratios did not differ between the patients taking or not taking these concomitant medications. In in vitro tests, none of the drugs tested, including the acetylcholinesterase inhibitors donepezil, galantamine, and tacrine, altered florbetapir F 18 binding to its target.
Use in Specific Populations
Pregnancy
- It is not known whether Amyvid can affect reproductive capacity or cause fetal harm when administered to a pregnant woman. Animal reproduction studies have not been conducted with Amyvid. Amyvid should be administered to a pregnant woman only if clearly needed.
- All radiopharmaceuticals, including Amyvid, have a potential to cause fetal harm. The likelihood of fetal harm depends on the stage of fetal development and the magnitude of the radiopharmaceutical dose. Assess pregnancy status before administering Amyvid to a female of reproductive potential.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Florbetapir F-18 in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Florbetapir F-18 during labor and delivery.
Nursing Mothers
- It is not known whether Amyvid is excreted in human milk. Because many drugs are excreted into human milk and because of the potential for radiation exposure to nursing infants from Amyvid, avoid use of the drug in a breastfeeding mother or have the mother temporarily interrupt breastfeeding for 24 hours (>10 half-lives of radioactive decay for the F 18 isotope) after exposure to Amyvid. If breastfeeding is interrupted, the patient should pump and discard her breast milk and use alternate infant nutrition sources (e.g., stored breast milk or infant formula) for 24 hours after administration of the drug.
Pediatric Use
- Amyvid is not indicated for use in pediatric patients.
Geriatic Use
- Of 496 patients in completed clinical studies of Amyvid, 307 patients were ≥65 years old (203 patients were over 75 years of age). No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Gender
There is no FDA guidance on the use of Florbetapir F-18 with respect to specific gender populations.
Race
There is no FDA guidance on the use of Florbetapir F-18 with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Florbetapir F-18 in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Florbetapir F-18 in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Florbetapir F-18 in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Florbetapir F-18 in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Florbetapir F-18 in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Florbetapir F-18 in the drug label.
Overdosage
There is limited information regarding Overdose of Florbetapir F-18 in the drug label.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox E numberTemplate:Chembox Supplement| Template:Chembox header2 | Florbetapir (18F) | |
|---|---|
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| |
| Properties | |
| C20H2518FN2O3 | |
| Molar mass | 359.4 g mol−1 |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
- Florbetapir F 18 binds to β-amyloid plaques and the F 18 isotope produces a positron signal that is detected by a PET scanner. In in vitro binding studies using postmortem human brain homogenates containing β-amyloid plaques, the dissociation constant (Kd) for florbetapir was 3.7 ± 0.3 nM. The binding of florbetapir F 18 to β-amyloid aggregates was demonstrated in postmortem human brain sections using autoradiographic methods, thioflavin S and traditional silver staining correlation studies as well as monoclonal antibody β-amyloid-specific correlation studies. Florbetapir binding to tau protein and a battery of neuroreceptors was not detected in in vitro studies.
Structure
- Following the intravenous administration of 370 MBq (10 mCi) of florbetapir F 18 to healthy volunteers, the drug was distributed throughout the body with less than 5% of the injected F 18 radioactivity present in the blood by 20 minutes following administration, and less than 2% present by 45 minutes after administration. The residual F 18 in circulation during the 30-90 minute imaging window was principally in the form of polar F 18 metabolites. Whole body scanning following the intravenous injection showed accumulation of radioactivity in the liver within four minutes post-injection, followed by elimination of the radioactivity predominantly through the biliary/gastrointestinal tract with much lower radioactivity detected in the bladder. Essentially all radioactivity collected in the urine was present as polar metabolites of florbetapir F 18.
- Amyvid is a sterile, non-pyrogenic radioactive diagnostic agent for intravenous injection. The clear, colorless solution is supplied ready to use and each milliliter contains 0.1 to 19 micrograms of florbetapir and 500 - 1900 MBq (13.5 - 51 mCi) florbetapir F 18 at EOS, 4.5 mg sodium ascorbate USP and 0.1 mL dehydrated alcohol USP in 0.9% sodium chloride injection USP. The pH of the solution is between 5.5 and 7.5.
Pharmacodynamics
- Following intravenous injection, florbetapir F 18 diffuses across the human blood-brain barrier and produces a radioactivity signal detectable throughout the brain. Subsequently, cerebral perfusion decreases the brain florbetapir F 18 content, with differential retention of the drug in areas that contain β-amyloid aggregates compared to areas that lack the aggregates. The time-activity curves for florbetapir F 18 in the brain of subjects with positive scans show continual signal increases from time zero through 30 minutes post-administration, with stable values thereafter up to at least 90 minutes post-injection. Differences in the signal intensity between portions of the brain that specifically retain florbetapir F 18 and the portions of the brain with nonspecific retention of the drug forms the image interpretation methods [see Dosage and Administration (2.4)].
- Clinical studies evaluated the test-retest distribution of florbetapir F 18 within the brains of 21 subjects (11 with probable AD and 10 healthy volunteers) who underwent two injections (with PET scans), separated by a time period of 2 to 30 days. Images were shown to maintain signal distribution reproducibility when evaluated qualitatively (by a reader masked to image time points) as well as quantitatively using an automated assessment of SUV in pre-specified brain regions. A comparison of a 10-minute image acquisition time versus a 20-minute acquisition time showed no difference in the mean cortical to cerebellar SUV ratio results obtained.
Pharmacokinetics
- Following the intravenous administration of 370 MBq (10 mCi) of florbetapir F 18 to healthy volunteers, the drug was distributed throughout the body with less than 5% of the injected F 18 radioactivity present in the blood by 20 minutes following administration, and less than 2% present by 45 minutes after administration. The residual F 18 in circulation during the 30-90 minute imaging window was principally in the form of polar F 18 metabolites. Whole body scanning following the intravenous injection showed accumulation of radioactivity in the liver within four minutes post-injection, followed by elimination of the radioactivity predominantly through the biliary/gastrointestinal tract with much lower radioactivity detected in the bladder. Essentially all radioactivity collected in the urine was present as polar metabolites of florbetapir F 18.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies to assess the carcinogenicity or reproductive toxicity potentials of Amyvid have not been conducted.
- In an in vitro bacterial reverse mutation assay (Ames test), increases in the number of revertant colonies were observed in 2 of the 5 strains exposed to 19F-AV-45, the non-radioactive form of florbetapir F 18. In a chromosomal aberration in vitro study with cultured human peripheral lymphocytes, 19F-AV-45 did not increase the percentage of cells with structural aberrations with 3-hour exposure with or without activation; however, 22-hour exposure produced a statistically significant increase in structural aberrations at all tested concentrations. Potential in vivo genotoxicity of 19F-AV-45 was evaluated in a rat micronucleus study. In this assay, 19F-AV-45 did not increase the number of micronucleated polychromatic erythrocytes at the highest achievable dose level, 372 μg/kg/day, when given twice daily for 3 consecutive days.
Clinical Studies
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies to assess the carcinogenicity or reproductive toxicity potentials of Amyvid have not been conducted.
- In an in vitro bacterial reverse mutation assay (Ames test), increases in the number of revertant colonies were observed in 2 of the 5 strains exposed to 19F-AV-45, the non-radioactive form of florbetapir F 18. In a chromosomal aberration in vitro study with cultured human peripheral lymphocytes, 19F-AV-45 did not increase the percentage of cells with structural aberrations with 3-hour exposure with or without activation; however, 22-hour exposure produced a statistically significant increase in structural aberrations at all tested concentrations. Potential in vivo genotoxicity of 19F-AV-45 was evaluated in a rat micronucleus study. In this assay, 19F-AV-45 did not increase the number of micronucleated polychromatic erythrocytes at the highest achievable dose level, 372 μg/kg/day, when given twice daily for 3 consecutive days.
- Study Two examined images only from terminally ill patients who had premortem Amyvid scans and postmortem brain examinations to determine a truth standard. Among the 59 patients, 35 of whom were also in Study One, the median age was 83 years (range 47 to 103 years), half were females and most were Caucasian (93%). Twenty-nine patients had an AD clinical diagnosis, 13 had another type of dementing disorder, 12 had no history of cognitive impairment and 5 had MCI. The time interval between the Amyvid scan and death was less than one year for 46 patients and between one and two years for 13 patients. Among the subset of patients who died within one year of Amyvid scanning (a prespecified outcome), the sensitivity using the majority interpretation of the readers was 96% (95% CI: 80% to 100%) and specificity was 100% (95% CI: 78% to 100%). With the entire dataset of 59 patients, the sensitivity using the majority interpretation of the readers was 92% (95% CI: 78% to 98%) and specificity was 100% (95% CI: 80% to 100%). At autopsy, the global brain neuritic plaque density category (CERAD score, as in TABLE 5) was: frequent n=30; moderate n=9; sparse n=5; and none n=15. TABLES 6 and 7 show the Amyvid performance characteristics among all the patients. Among the subset of patients who died within one year of Amyvid scanning (n=46; 28 positive and 18 negative based on histopathology) the median (and range) of correct read results, false negatives, and false positives were 44 (37 to 45), 1 (0 to 7), and 1 (0 to 2), respectively, for In-Person Training (Study Two); and were 43 (38 to 44), 3 (0 to 7), and 1 (0 to 2), respectively, for Electronic Media Training (Study Three).
- Study Three included images from subjects who did not have a truth standard (20 healthy volunteers, 52 patients with mild cognitive impairment, 20 patients with AD) as well as all 59 of the patients who underwent an autopsy (same patients as in Study Two) and provided a truth standard. Duplicate images of 33 patients were included within the total pool of images in order to assess intra-reader image reproducibility. Among the 151 subjects, the median age was 76 years (range 47 to 103), half were females and most were Caucasian (93.4%). Performance characteristics for patients with a truth standard are shown above (TABLES 6 and 7). The major reproducibility results are shown in TABLE 8 for various groups of subjects. Inter-reader reproducibility analyses for all images showed an overall Fleiss' kappa statistic of 0.83 (95% CI: 0.78 to 0.88); the lower bound of the 95% CI exceeded the pre-specified success criterion (95% CI lower bound >0.58). Intra-reader reproducibility analyses showed that, between the two readings for each of the 33 patients with duplicate images, one of the five readers had complete agreement for all 33 patients, two readers had discrepant reads for a single patient, one reader had discrepant reads for two patients and another reader had discrepant reads for three patients.
How Supplied
- Amyvid is supplied in 10 mL, 30 mL, or 50 mL vials containing 10 mL, 10-30 mL, or 10-50 mL, respectively, of a clear, colorless solution at a strength of 500 - 1900 MBq/mL (13.5 - 51 mCi/mL) florbetapir F 18 at EOS. Each vial contains multiple doses and is enclosed in a shielded container to minimize external radiation exposure.
- 10 mL NDC 0002-1200-10 (IC1200)
- 30 mL NDC 0002-1200-30 (IC1200)
- 50 mL NDC 0002-1200-50 (IC1200)
Storage
- Store Amyvid at 25ºC (77°F); excursions permitted to 15ºC to 30ºC (59°F to 86°F) [see USP Controlled Room Temperature]. The product does not contain a preservative. Store Amyvid within the original container or equivalent radiation shielding. Amyvid must not be diluted.
- This preparation is approved for use by persons under license by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
Images
Drug Images
{{#ask: Page Name::Florbetapir F-18 |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL

This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
Ingredients and Appearance
{{#ask: Label Page::Florbetapir F-18 |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Instruct patients to inform their physician or healthcare provider if they are pregnant or breastfeeding.
Inform patients who are breastfeeding to use alternate infant nutrition sources (e.g., stored breast milk or infant formula) for 24 hours (>10 half-lives of radioactive decay for the F 18 isotope) after administration of the drug or avoid use of the drug.
Precautions with Alcohol
- Alcohol-Florbetapir F-18 interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Amyvid®[1]
Look-Alike Drug Names
There is limited information regarding the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.