Ertugliflozin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ertugliflozin is a sodium glucose co-transporter 2 (SGLT2) inhibitor that is FDA approved for the adjunct of diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Common adverse reactions include female genital mycotic infections.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Ertugliflozin is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use
- Ertugliflozin is not recommended in patients with type 1 diabetes mellitus or for the treatment of diabetic ketoacidosis.
Recommended Dosage
- The recommended starting dose of ertugliflozin is 5 mg once daily, taken in the morning, with or without food. In patients tolerating ertugliflozin 5 mg once daily, the dose may be increased to a maximum recommended dose of 15 mg once daily if additional glycemic control is needed.
- In patients with volume depletion, correct this condition prior to initiation of ertugliflozin.
Patients with Renal Impairment
- Assess renal function prior to initiation of ertugliflozin and periodically thereafter.
- Use of ertugliflozin is contraindicated in patients with an eGFR less than 30 mL/minute/1.73 m2.
- Initiation of ertugliflozin is not recommended in patients with an eGFR of 30 mL/minute/1.73 m2 to less than 60 mL/minute/1.73 m2.
- Continued use of ertugliflozin is not recommended when eGFR is persistently between 30 and less than 60 mL/minute/1.73 m2.
- No dose adjustment is needed in patients with mild renal impairment.
Dosage Forms and Strengths
- Tablets: 5 mg, pink, triangular-shaped debossed with "701" on one side and plain on the other side.
- Tablets: 15 mg, red, triangular-shaped debossed with "702" on one side and plain on the other side.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding ertugliflozin Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding ertugliflozin Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ertugliflozin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding ertugliflozin Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding ertugliflozin Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Severe renal impairment, end-stage renal disease (ESRD), or dialysis.
- History of a serious hypersensitivity reaction to ertugliflozin.
Warnings
Hypotension
- Ertugliflozin causes intravascular volume contraction. Therefore, symptomatic hypotension may occur after initiating ertugliflozin particularly in patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2) [see USE IN SPECIFIC POPULATIONS (8.6)], elderly patients (≥65 years), in patients with low systolic blood pressure, and in patients on diuretics. Before initiating ertugliflozin, volume status should be assessed and corrected if indicated. Monitor for signs and symptoms of hypotension after initiating therapy.
Ketoacidosis
- Reports of ketoacidosis, a serious life-threatening condition requiring urgent hospitalization, have been identified in clinical trials and postmarketing surveillance in patients with type 1 and type 2 diabetes mellitus receiving sodium glucose co-transporter-2 (SGLT2) inhibitors and cases have been reported in ertugliflozin-treated patients in clinical trials. Across the clinical program, ketoacidosis was identified in 3 of 3,409 (0.1%) of ertugliflozin-treated patients and 0% of comparator-treated patients. Fatal cases of ketoacidosis have been reported in patients taking SGLT2 inhibitors. Ertugliflozin is not indicated for the treatment of patients with type 1 diabetes mellitus.
- Patients treated with ertugliflozin who present with signs and symptoms consistent with severe metabolic acidosis should be assessed for ketoacidosis regardless of presenting blood glucose levels, as ketoacidosis associated with ertugliflozin may be present even if blood glucose levels are less than 250 mg/dL. If ketoacidosis is suspected, ertugliflozin should be discontinued, patient should be evaluated, and prompt treatment should be instituted. Treatment of ketoacidosis may require insulin, fluid and carbohydrate replacement.
- In many of the reported cases, and particularly in patients with type 1 diabetes, the presence of ketoacidosis was not immediately recognized and institution of treatment was delayed because presenting blood glucose levels were below those typically expected for diabetic ketoacidosis (often less than 250 mg/dL). Signs and symptoms at presentation were consistent with dehydration and severe metabolic acidosis and included nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. In some but not all cases, factors predisposing to ketoacidosis such as insulin dose reduction, acute febrile illness, reduced caloric intake due to illness or surgery, pancreatic disorders suggesting insulin deficiency (e.g., type 1 diabetes, history of pancreatitis or pancreatic surgery), and alcohol abuse were identified.
- Before initiating ertugliflozin, consider factors in the patient history that may predispose to ketoacidosis, including pancreatic insulin deficiency from any cause, caloric restriction, and alcohol abuse. In patients treated with ertugliflozin consider monitoring for ketoacidosis and temporarily discontinuing ertugliflozin in clinical situations known to predispose to ketoacidosis (e.g., prolonged fasting due to acute illness or surgery).
Acute Kidney Injury and Impairment in Renal Function
- Ertugliflozin causes intravascular volume contraction and can cause renal impairment. There have been postmarketing reports of acute kidney injury some requiring hospitalization and dialysis in patients receiving SGLT2 inhibitors.
- Before initiating ertugliflozin, consider factors that may predispose patients to acute kidney injury including hypovolemia, chronic renal insufficiency, congestive heart failure and concomitant medications (diuretics, ACE inhibitors, ARBs, NSAIDs). Consider temporarily discontinuing ertugliflozin in any setting of reduced oral intake (such as acute illness or fasting) or fluid losses (such as gastrointestinal illness or excessive heat exposure); monitor patients for signs and symptoms of acute kidney injury. If acute kidney injury occurs, discontinue ertugliflozin promptly and institute treatment.
- Ertugliflozin increases serum creatinine and decreases eGFR. Patients with moderate renal impairment (eGFR 30 to less than 60 mL/min/1.73 m2) may be more susceptible to these changes. Renal function abnormalities can occur after initiating ertugliflozin. Renal function should be evaluated prior to initiating ertugliflozin and periodically thereafter. Use of ertugliflozin is not recommended when eGFR is persistently between 30 and less than 60 mL/min/1.73 m2 and is contraindicated in patients with an eGFR less than 30 mL/min/1.73 m2
Urosepsis and Pyelonephritis
- There have been postmarketing reports of serious urinary tract infections, including urosepsis and pyelonephritis, requiring hospitalization in patients receiving SGLT2 inhibitors. Cases of pyelonephritis also have been reported in ertugliflozin-treated patients in clinical trials. Treatment with SGLT2 inhibitors increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated.
Lower Limb Amputation
- An increased risk for lower limb amputation (primarily of the toe) has been observed in clinical studies with another SGLT2 inhibitor. Across seven Phase 3 clinical trials in the ertugliflozin development program, non-traumatic lower limb amputations were reported in 1 (0.1%) patient in the comparator group, 3 (0.2%) patients in the ertugliflozin 5 mg group, and 8 (0.5%) patients in the ertugliflozin 15 mg group. A causal association between ertugliflozin and lower limb amputation has not been definitively established.
- Before initiating ertugliflozin, consider factors in the patient history that may predispose them to the need for amputations, such as a history of prior amputation, peripheral vascular disease, neuropathy and diabetic foot ulcers. Counsel patients about the importance of routine preventative foot care. Monitor patients receiving ertugliflozin for signs and symptoms of infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and discontinue ertugliflozin if these complications occur.
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
- Insulin and insulin secretagogues (e.g., sulfonylurea) are known to cause hypoglycemia. ertugliflozin may increase the risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with ertugliflozin.
Genital Mycotic Infections
- Ertugliflozin increases the risk of genital mycotic infections. Patients who have a history of genital mycotic infections or who are uncircumcised are more likely to develop genital mycotic infections. Monitor and treat appropriately.
Increases in Low-Density Lipoprotein Cholesterol (LDL-C)
- Dose-related increases in LDL-C can occur with ertugliflozin. Monitor and treat as appropriate.
Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with ertugliflozin.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials Evaluating Ertugliflozin 5 and 15 mg
- The data in Table 1 are derived from a pool of three 26-week, placebo-controlled trials. Ertugliflozin was used as monotherapy in one trial and as add-on therapy in two trials. These data reflect exposure of 1,029 patients to ertugliflozin with a mean exposure duration of approximately 25 weeks. Patients received ertugliflozin 5 mg (N=519), ertugliflozin 15 mg (N=510), or placebo (N=515) once daily. The mean age of the population was 57 years and 2% were older than 75 years of age. Fifty-three percent (53%) of the population was male and 73% were Caucasian, 15% were Asian, and 7% were Black or African American. At baseline the population had diabetes for an average of 7.5 years, had a mean HbA1c of 8.1%, and 19.4% had established microvascular complications of diabetes. Baseline renal function (mean eGFR 88.9 mL/min/1.73 m2) was normal or mildly impaired in 97% of patients and moderately impaired in 3% of patients.
- Table 1 shows common adverse reactions associated with the use of ertugliflozin. These adverse reactions were not present at baseline, occurred more commonly on ertugliflozin than on placebo, and occurred in at least 2% of patients treated with either ertugliflozin 5 mg or ertugliflozin 15 mg.

Volume Depletion
- Ertugliflozin causes an osmotic diuresis, which may lead to intravascular volume contraction and adverse reactions related to volume depletion, particularly in patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2). In patients with moderate renal impairment, adverse reactions related to volume depletion (e.g., dehydration, dizziness postural, presyncope, syncope, hypotension, and orthostatic hypotension) were reported in 0%, 4.4%, and 1.9% of patients treated with placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively. Ertugliflozin may also increase the risk of hypotension in other patients at risk for volume contraction.
Ketoacidosis
- Across the clinical program, ketoacidosis was identified in 3 of 3,409 (0.1%) ertugliflozin-treated patients and 0.0% of comparator-treated patients
Impairment in Renal Function
- Treatment with ertugliflozin was associated with increases in serum creatinine and decreases in eGFR (see TABLE 2). Patients with moderate renal impairment at baseline had larger mean changes. In a study in patients with moderate renal impairment, these abnormal laboratory findings were observed to reverse after treatment discontinuation.

- Renal-related adverse reactions (e.g., acute kidney injury, renal impairment, acute prerenal failure) may occur in patients treated with ertugliflozin, particularly in patients with moderate renal impairment where the incidence of renal-related adverse reactions was 0.6%, 2.5%, and 1.3% in patients treated with placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively.
Lower Limb Amputation
- Across seven Phase 3 clinical trials in which ertugliflozin was studied as monotherapy and in combination with other antihyperglycemic agents, non-traumatic lower limb amputations occurred in 1 of 1,450 (0.1%) in the non-ertugliflozin group, 3 of 1,716 (0.2%) in the ertugliflozin 5 mg group, and 8 of 1,693 (0.5%) in the ertugliflozin 15 mg group.
Hypoglycemia
- The incidence of hypoglycemia by study is shown in Table 3.

Genital Mycotic Infections
- In the pool of three placebo-controlled clinical trials, the incidence of female genital mycotic infections (e.g., genital candidiasis, genital infection fungal, vaginal infection, vulvitis, vulvovaginal candidiasis, vulvovaginal mycotic infection, vulvovaginitis) occurred in 3%, 9.1%, and 12.2% of females treated with placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively (see TABLE 1). In females, discontinuation due to genital mycotic infections occurred in 0% and 0.6% of patients treated with placebo and ertugliflozin, respectively.
- In the same pool, male genital mycotic infections (e.g., balanitis candida, balanoposthitis, genital infection, genital infection fungal) occurred in 0.4%, 3.7%, and 4.2% of males treated with placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively (see TABLE 1). Male genital mycotic infections occurred more commonly in uncircumcised males. In males, discontinuations due to genital mycotic infections occurred in 0% and 0.2% of patients treated with placebo and ertugliflozin, respectively. Phimosis was reported in 8 of 1729 (0.5%) male ertugliflozin-treated patients, of which four required circumcision.
Laboratory Tests
Increases in Low-Density Lipoprotein Cholesterol (LDL-C)
- In the pool of three placebo-controlled trials, dose-related increases in LDL-C were observed in patients treated with ertugliflozin. Mean percent changes from baseline to Week 26 in LDL-C relative to placebo were 2.6% and 5.4% with ertugliflozin 5 mg and ertugliflozin 15 mg, respectively. The range of mean baseline LDL-C was 96.6 to 97.7 mg/dL across treatment groups.
Increases in Hemoglobin
- In the pool of three placebo-controlled trials, mean changes (percent changes) from baseline to Week 26 in hemoglobin were -0.21 g/dL (-1.4%) with placebo, 0.46 g/dL (3.5%) with ertugliflozin 5 mg, and 0.48 g/dL (3.5%) with ertugliflozin 15 mg. The range of mean baseline hemoglobin was 13.90 to 14.00 g/dL across treatment groups. At the end of treatment, 0.0%, 0.2%, and 0.4% of patients treated with placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively, had a hemoglobin increase greater than 2 g/dL and above the upper limit of normal.
Increases in Serum Phosphate
- In the pool of three placebo-controlled trials, mean changes (percent changes) from baseline in serum phosphate were 0.04 mg/dL (1.9%) with placebo, 0.21 mg/dL (6.8%) with ertugliflozin 5 mg, and 0.26 mg/dL (8.5%) with ertugliflozin 15 mg. The range of mean baseline serum phosphate was 3.53 to 3.54 mg/dL across treatment groups. In a clinical trial of patients with moderate renal impairment, mean changes (percent changes) from baseline at Week 26 in serum phosphate were -0.01 mg/dL (0.8%) with placebo, 0.29 mg/dL (9.7%) with ertugliflozin 5 mg, and 0.24 mg/dL (7.8%) with ertugliflozin 15 mg.
Postmarketing Experience
There is limited information regarding Ertugliflozin Postmarketing Experience in the drug label.
Drug Interactions
- Concomitant Use with Insulin and Insulin Secretagogues
- Positive Urine Glucose Test
- Interference with 1,5-anhydroglucitol (1,5-AG) Assay
Concomitant Use with Insulin and Insulin Secretagogues
- Ertugliflozin may increase the risk of hypoglycemia when used in combination with insulin and/or an insulin secretagogue. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with ertugliflozin.
Positive Urine Glucose Test
- Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors as SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Use alternative methods to monitor glycemic control.
Interference with 1,5-anhydroglucitol (1,5-AG) Assay
- Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.
Use in Specific Populations
Pregnancy
Risk Summary
- Based on animal data showing adverse renal effects, ertugliflozin is not recommended during the second and third trimesters of pregnancy.
- The limited available data with ertugliflozin in pregnant women are not sufficient to determine a drug-associated risk of adverse developmental outcomes. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy.
- In animal studies, adverse renal changes were observed in rats when ertugliflozin was administered during a period of renal development corresponding to the late second and third trimesters of human pregnancy. Doses approximately 13 times the maximum clinical dose caused renal pelvic and tubule dilatations and renal mineralization that were not fully reversible. There was no evidence of fetal harm in rats or rabbits at exposures of ertugliflozin approximately 300 times higher than the maximal clinical dose of 15 mg/day when administered during organogenesis.
- The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
- Poorly-controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, stillbirth, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data (Animal)
- When ertugliflozin was orally administered to juvenile rats from PND 21 to PND 90, increased kidney weight, renal tubule and renal pelvis dilatation, and renal mineralization occurred at doses greater than or equal to 5 mg/kg (13-fold human exposures, based on AUC). These effects occurred with drug exposure during periods of renal development in rats that correspond to the late second and third trimester of human renal development, and did not fully reverse within a 1-month recovery period.
- In embryo-fetal development studies, ertugliflozin (50, 100 and 250 mg/kg/day) was administered orally to rats on gestation days 6 to 17 and to rabbits on gestation days 7 to 19. Ertugliflozin did not adversely affect developmental outcomes in rats and rabbits at maternal exposures that were approximately 300 times the human exposure at the maximum clinical dose of 15 mg/day, based on AUC. A maternally toxic dose (250 mg/kg/day) in rats (707 times the clinical dose), was associated with reduced fetal viability, and a higher incidence of a visceral malformation (membranous ventricular septal defect). In the pre- and post-natal development study in pregnant rats, ertugliflozin was administered to the dams from gestation day 6 through lactation day 21 (weaning). Decreased post-natal growth (weight gain) was observed at maternal doses ≥100 mg/kg/day (greater than or equal to 331 times the human exposure at the maximum clinical dose of 15 mg/day, based on AUC).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ertugliflozin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ertugliflozin during labor and delivery.
Nursing Mothers
Risk Summary
- There is no information regarding the presence of ertugliflozin in human milk, the effects on the breastfed infant, or the effects on milk production. Ertugliflozin is present in the milk of lactating rats. Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney. Because of the potential for serious adverse reactions in a breastfed infant, advise women that the use of ertugliflozin is not recommended while breastfeeding.
Data (Animal)
- The lacteal excretion of radiolabeled ertugliflozin in lactating rats was evaluated 10 to 12 days after parturition. Ertugliflozin derived radioactivity exposure in milk and plasma were similar, with a milk/plasma ratio of 1.07, based on AUC. Juvenile rats directly exposed to ertugliflozin during a developmental period corresponding to human kidney maturation were associated with a risk to the developing kidney (persistent increased organ weight, renal mineralization, and renal pelvic and tubular dilatations).
Pediatric Use
- Safety and effectiveness of ertugliflozin in pediatric patients under 18 years of age have not been established.
Geriatic Use
- No dosage adjustment of ertugliflozin is recommended based on age. Across the clinical program, a total of 876 (25.7%) patients treated with ertugliflozin were 65 years and older, and 152 (4.5%) patients treated with ertugliflozin were 75 years and older. Patients 65 years and older had a higher incidence of adverse reactions related to volume depletion compared to younger patients; events were reported in 1.1%, 2.2%, and 2.6% of patients treated with comparator, ertugliflozin 5 mg, and ertugliflozin 15 mg, respectively. Ertugliflozin is expected to have diminished efficacy in elderly patients with renal impairment.
Gender
There is no FDA guidance on the use of Ertugliflozin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ertugliflozin with respect to specific racial populations.
Renal Impairment
- The safety and efficacy of ertugliflozin have not been established in patients with type 2 diabetes mellitus and moderate renal impairment. Compared to placebo-treated patients, patients with moderate renal impairment treated with ertugliflozin did not have improvement in glycemic control, and had increased risks for renal impairment, renal-related adverse reactions and volume depletion adverse reactions. Therefore, ertugliflozin is not recommended in this population.
- Ertugliflozin is contraindicated in patients with severe renal impairment, ESRD, or receiving dialysis. Ertugliflozin is not expected to be effective in these patient populations.
- No dosage adjustment or increased monitoring is needed in patients with mild renal impairment.
Hepatic Impairment
- No dosage adjustment of ertugliflozin is necessary in patients with mild or moderate hepatic impairment. Ertugliflozin has not been studied in patients with severe hepatic impairment and is not recommended for use in this patient population.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ertugliflozin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ertugliflozin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Assess glycemic control, including target HbA1c goal; use alternative methods to urine glucose testing and 1,5-anhydroglucitol assay, which may result in false positive results.
- HbA1c: Twice yearly in patients who are meeting treatment goals; every 3 months in patients whose therapy has changed and/or who are not meeting glycemic goals; more frequently as clinically warranted.
- Renal function: Prior to initiation and periodically during therapy.
- LL-C levels.
- Genital mycotic infections: Especially in those with a history of genital mycotic infections or who are uncircumcised.
- Lower limbs: For signs and symptoms of infection, including osteomyelitis, new pain or tenderness, and sores or ulcers; especially in patients with a history of prior amputation, peripheral vascular disease, neuropathy, or diabetic foot ulcers.
- Signs and symptoms of acute kidney injury: Especially in patients at risk including hypovolemia, chronic renal insufficiency, congestive heart failure, and concomitant use of diuretics, ACE inhibitors, angiotensin receptor blockers, or NSAIDs.
- Signs and symptoms of hypotension: Especially in patients with renal impairment (estimated GFR less than 60mL/min/1.73 m(2)), geriatric patients (65 years or older), those with low systolic blood pressure, and concomitant use of diuretics.
- Signs and symptoms of ketoacidosis: Especially in patients presenting with metabolic acidosis, regardless of blood glucose levels.
- Signs and symptoms of urinary tract infection.
- Volume status: Prior to initiation of therapy.
IV Compatibility
There is limited information regarding the compatibility of Ertugliflozin and IV administrations.
Overdosage
- In the event of an overdose with ertugliflozin, contact the Poison Control Center. Employ the usual supportive measures as dictated by the patient's clinical status. Removal of ertugliflozin by hemodialysis has not been studied.
Pharmacology
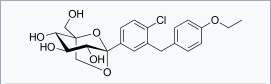
| |
Ertugliflozin
| |
| Systematic (IUPAC) name | |
| (1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)phenyl]-1-(hydroxymethyl)-6,8-dioxabicyclo[3.2.1]octane-2,3,4-triol | |
| Identifiers | |
| CAS number | |
| ATC code | None |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Synonyms | PF-04971729 |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | 93.6% |
| Metabolism | UGT1A9, UGT2B7 |
| Half life | ~17 hours |
| Excretion | 41% faeces, 50% urine |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
?(CA) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth |
Mechanism of Action
- SGLT2 is the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. Ertugliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, ertugliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Structure

Pharmacodynamics
Urinary Glucose Excretion and Urinary Volume
- Dose-dependent increases in the amount of glucose excreted in urine were observed in healthy subjects and in patients with type 2 diabetes mellitus following single- and multiple-dose administration of ertugliflozin. Dose-response modeling indicates that ertugliflozin 5 mg and 15 mg result in near maximal urinary glucose excretion (UGE). Enhanced UGE is maintained after multiple-dose administration. UGE with ertugliflozin also results in increases in urinary volume.
Cardiac Electrophysiology
- The effect of ertugliflozin on QTc interval was evaluated in a Phase 1 randomized, placebo- and positive-controlled 3-period crossover study in 42 healthy subjects. At 6.7 times the therapeutic exposures with maximum recommended dose, ertugliflozin does not prolong QTc to any clinically relevant extent.
Pharmacokinetics
- The pharmacokinetics of ertugliflozin are similar in healthy subjects and patients with type 2 diabetes mellitus. The steady state mean plasma AUC and Cmax were 398 ng∙hr/mL and 81.3 ng/mL, respectively, with 5 mg ertugliflozin once-daily treatment, and 1,193 ng∙hr/mL and 268 ng/mL, respectively, with 15 mg ertugliflozin once-daily treatment. Steady-state is reached after 4 to 6 days of once-daily dosing with ertugliflozin. Ertugliflozin does not exhibit time-dependent pharmacokinetics and accumulates in plasma up to 10-40% following multiple dosing.
Absorption
- Following single-dose oral administration of 5 mg and 15 mg of ertugliflozin, peak plasma concentrations (median Tmax) of ertugliflozin occur at 1 hour postdose under fasted conditions. Plasma Cmax and AUC of ertugliflozin increase in a dose-proportional manner following single doses from 0.5 mg (0.1 times the lowest recommended dose) to 300 mg (20 times the highest recommended dose) and following multiple doses from 1 mg (0.2 times the lowest recommended dose) to 100 mg (6.7 times the highest recommended dose). The absolute oral bioavailability of ertugliflozin following administration of a 15 mg dose is approximately 100%.
Effect of Food
- Administration of ertugliflozin with a high-fat and high-calorie meal decreases ertugliflozin Cmax by 29% and prolongs Tmax by 1 hour, but does not alter AUC as compared with the fasted state. The observed effect of food on ertugliflozin pharmacokinetics is not considered clinically relevant, and ertugliflozin may be administered with or without food. In Phase 3 clinical trials, ertugliflozin was administered without regard to meals.
Distribution
- The mean steady-state volume of distribution of ertugliflozin following an intravenous dose is 85.5 L. Plasma protein binding of ertugliflozin is 93.6% and is independent of ertugliflozin plasma concentrations. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment. The blood-to-plasma concentration ratio of ertugliflozin is 0.66.
Elimination
Metabolism
- Metabolism is the primary clearance mechanism for ertugliflozin. The major metabolic pathway for ertugliflozin is UGT1A9 and UGT2B7-mediated O-glucuronidation to two glucuronides that are pharmacologically inactive at clinically relevant concentrations. CYP-mediated (oxidative) metabolism of ertugliflozin is minimal (12%).
Excretion
- The mean systemic plasma clearance following an intravenous 100 µg dose was 11.2 L/hr. The mean elimination half-life in type 2 diabetic patients with normal renal function was estimated to be 16.6 hours based on the population pharmacokinetic analysis. Following administration of an oral [14C]-ertugliflozin solution to healthy subjects, approximately 40.9% and 50.2% of the drug-related radioactivity was eliminated in feces and urine, respectively. Only 1.5% of the administered dose was excreted as unchanged ertugliflozin in urine and 33.8% as unchanged ertugliflozin in feces, which is likely due to biliary excretion of glucuronide metabolites and subsequent hydrolysis to parent.
Specific Populations
Patients with Renal Impairment
- In a Phase 1 clinical pharmacology study in patients with type 2 diabetes mellitus and mild, moderate, or severe renal impairment (as determined by eGFR), following a single-dose administration of 15 mg ertugliflozin, the mean increases in AUC of ertugliflozin were 1.6-, 1.7-, and 1.6-fold, respectively, for mild, moderate and severe renally impaired patients, compared to subjects with normal renal function. These increases in ertugliflozin AUC are not considered clinically meaningful. The 24-hour urinary glucose excretion declined with increasing severity of renal impairment. The plasma protein binding of ertugliflozin was unaffected in patients with renal impairment.
Patients with Hepatic Impairment
- Moderate hepatic impairment (based on the Child-Pugh classification) did not result in an increase in exposure of ertugliflozin. The AUC of ertugliflozin decreased by approximately 13%, and Cmax decreased by approximately 21% compared to subjects with normal hepatic function. This decrease in ertugliflozin exposure is not considered clinically meaningful. There is no clinical experience in patients with Child-Pugh class C (severe) hepatic impairment. The plasma protein binding of ertugliflozin was unaffected in patients with moderate hepatic impairment.
Pediatric Patients
- No studies with ertugliflozin have been performed in pediatric patients.
Effects of Age, Body Weight, Gender, and Race
- Based on a population pharmacokinetic analysis, age, body weight, gender, and race do not have a clinically meaningful effect on the pharmacokinetics of ertugliflozin.
Drug Interaction Studies
In Vitro Assessment of Drug Interactions
- In in vitro studies, ertugliflozin and ertugliflozin glucuronides did not inhibit CYP450 isoenzymes (CYPs) 1A2, 2C9, 2C19, 2C8, 2B6, 2D6, or 3A4, and did not induce CYPs 1A2, 2B6, or 3A4. Ertugliflozin was not a time-dependent inhibitor of CYP3A in vitro. Ertugliflozin did not inhibit UGT1A6, 1A9, or 2B7 in vitro and was a weak inhibitor (IC5039 µM) of UGT1A1 and 1A4. Ertugliflozin glucuronides did not inhibit UGT1A1, 1A4, 1A6, 1A9, or 2B7 in vitro. Overall, ertugliflozin is unlikely to affect the pharmacokinetics of drugs eliminated by these enzymes. Ertugliflozin is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) transporters and is not a substrate of organic anion transporters (OAT1, OAT3), organic cation transporters (OCT1, OCT2), or organic anion transporting polypeptides (OATP1B1, OATP1B3). Ertugliflozin or ertugliflozin glucuronides do not meaningfully inhibit P-gp, OCT2, OAT1, or OAT3 transporters, or transporting polypeptides OATP1B1 and OATP1B3, at clinically relevant concentrations. Overall, ertugliflozin is unlikely to affect the pharmacokinetics of concurrently administered medications that are substrates of these transporters.
In Vivo Assessment of Drug Interactions
- No dose adjustment of ertugliflozin is recommended when coadministered with commonly prescribed medicinal products. Ertugliflozin pharmacokinetics were similar with and without coadministration of metformin, glimepiride, sitagliptin, and simvastatin in healthy subjects (see FIGURE 1). Coadministration of ertugliflozin with multiple doses of 600 mg once-daily rifampin (an inducer of UGT and CYP enzymes) resulted in approximately 39% and 15% mean reductions in ertugliflozin AUC and Cmax, respectively, relative to ertugliflozin administered alone. These changes in exposure are not considered clinically relevant. Ertugliflozin had no clinically relevant effect on the pharmacokinetics of metformin, glimepiride, sitagliptin, and simvastatin when coadministered in healthy subjects (see FIGURE 2). Physiologically-based PK (PBPK) modeling suggests that coadministration of mefenamic acid (UGT inhibitor) may increase the AUC and Cmax of ertugliflozin by 1.51- and 1.19-fold, respectively. These predicted changes in exposure are not considered clinically relevant.

Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Carcinogenicity was evaluated in CD-1 mice and Sprague-Dawley rats. In the mouse study, ertugliflozin was administered by oral gavage at doses of 5, 15, and 40 mg/kg/day for up to 97 weeks in males and 102 weeks in females. There were no ertugliflozin-related neoplastic findings at doses up to 40 mg/kg/day (approximately 50 times human exposure at the maximum recommended human dose [MRHD] of 15 mg/day based on AUC). In the rat study, ertugliflozin was administered by oral gavage at doses of 1.5, 5, and 15 mg/kg/day for up to 92 weeks in females and 104 weeks in males. Ertugliflozin-related neoplastic findings included an increased incidence of adrenal medullary pheochromocytoma (PCC) in male rats at 15 mg/kg/day. Although the molecular mechanism remains unknown, this finding may be related to carbohydrate malabsorption leading to altered calcium homeostasis, which has been associated with PCC development in rats and has unclear relevancy to human risk. The no-observed-effect level (NOEL) for neoplasia was 5 mg/kg/day (approximately 16 times human exposure at the MRHD of 15 mg/day, based on AUC).
Mutagenesis
- Ertugliflozin was not mutagenic or clastogenic with or without metabolic activation in the microbial reverse mutation, in vitro cytogenetic (human lymphocytes), and in vivo rat micronucleus assays.
Impairment of Fertility
- In the rat fertility and embryonic development study, male and female rats were administered ertugliflozin at 5, 25, and 250 mg/kg/day. No effects on fertility were observed at 250 mg/kg/day (approximately 480 and 570 times male and female human exposures, respectively, at the MRHD of 15 mg/day based on AUC comparison).
Clinical Studies
Overview of Clinical Studies in Patients with Type 2 Diabetes Mellitus
- The efficacy and safety of ertugliflozin have been studied in 7 multicenter, randomized, double-blind, placebo- or active comparator-controlled, clinical studies involving 4,863 patients with type 2 diabetes mellitus. These studies included White, Hispanic, Black, Asian, and other racial and ethnic groups, and patients with an average age of approximately 57.8 years.
- Ertugliflozin has been studied as monotherapy and in combination with metformin and/or a dipeptidyl peptidase 4 (DPP-4) inhibitor. Ertugliflozin has also been studied in combination with antidiabetic medications, including insulin and a sulfonylurea, in patients with type 2 diabetes mellitus with moderate renal impairment.
- In patients with type 2 diabetes mellitus treatment with ertugliflozin reduced hemoglobin A1c (HbA1c) compared to placebo.
- In patients with type 2 diabetes mellitus treated with ertugliflozin, the reduction in HbA1c was generally similar across subgroups defined by age, sex, race, geographic region, baseline body mass index (BMI), and duration of type 2 diabetes mellitus. In patients with type 2 diabetes mellitus and moderate renal impairment, treatment with ertugliflozin did not result in a reduction in HbA1c compared to placebo.
Clinical Study of Monotherapy Use of Ertugliflozin in Patients with Type 2 Diabetes Mellitus
- A total of 461 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on diet and exercise participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT01958671) to evaluate the efficacy and safety of ertugliflozin monotherapy. These patients, who were either treatment naïve or not receiving any background antihyperglycemic treatment ≥8 weeks, entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, ertugliflozin 5 mg, or ertugliflozin 15 mg, administered once daily.
- At Week 26, treatment with ertugliflozin at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo. Ertugliflozin also resulted in a greater proportion of patients achieving an HbA1c <7% compared with placebo (see TABLE 4 and FIGURE 3).

- The mean baseline body weight was 94.2 kg, 94.0 kg, and 90.6 kg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.0 kg, -3.0 kg, and -3.1 kg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The difference from placebo (95% CI) for ertugliflozin 5 mg was -2.0 kg (-2.8, -1.2) and for ertugliflozin 15 mg was -2.1 kg (-2.9, -1.3).

Clinical Studies of Combination Therapy Use of Ertugliflozin in Patients with Type 2 Diabetes Mellitus
Add-on Combination Therapy with Metformin
- A total of 621 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on metformin monotherapy (≥1,500 mg/day for ≥8 weeks) participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT02033889) to evaluate the efficacy and safety of ertugliflozin in combination with metformin. Patients entered a 2-week, single-blind, placebo run-in, and were randomized to placebo, ertugliflozin 5 mg, or ertugliflozin 15 mg administered once daily in addition to continuation of background metformin therapy.
- At Week 26, treatment with ertugliflozin at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c compared to placebo. Ertugliflozin also resulted in a greater proportion of patients achieving an HbA1c <7% compared to placebo (see TABLE 5).

- The mean baseline body weight was 84.5 kg, 84.9 kg, and 85.3 kg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.4 kg, -3.2 kg, and -3.0 kg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The difference from placebo (95% CI) for ertugliflozin 5 mg was -1.8 kg (-2.4, -1.2) and for ertugliflozin 15 mg was -1.7 kg (-2.2, -1.1).
- The mean baseline systolic blood pressure was 129.3 mmHg, 130.5 mmHg, and 130.2 mmHg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.8 mmHg, -5.1 mmHg, and -5.7 mmHg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The difference from placebo (95% CI) for ertugliflozin 5 mg was -3.3 mmHg (-5.6, -1.1) and for ertugliflozin 15 g was -3.8 mmHg (-6.1, -1.5).
Active Controlled Study versus Glimepiride as Add-on Combination Therapy with Metformin
- A total of 1,326 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 9%) on metformin monotherapy participated in a randomized, double-blind, multi-center, 52-week, active comparator controlled study (NCT01999218) to evaluate the efficacy and safety of ertugliflozin in combination with metformin. These patients, who were receiving metformin monotherapy (≥1,500 mg/day for ≥8 weeks), entered a 2-week, single-blind, placebo run-in period and were randomized to glimepiride, ertugliflozin 5 mg, or ertugliflozin 15 mg administered once daily in addition to continuation of background metformin therapy. Glimepiride was initiated at 1 mg/day and titrated up to a maximum dose of 6 or 8 mg/day (depending on maximum approved dose in each country) or a maximum tolerated dose or down-titrated to avoid or manage hypoglycemia. The mean daily dose of glimepiride was 3.0 mg.
- Ertugliflozin 15 mg was non-inferior to glimepiride after 52 weeks of treatment. (See TABLE 6.)

- The mean baseline body weight was 86.8 kg, 87.9 kg, and 85.6 kg in the glimepiride, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The mean changes from baseline to Week 52 were 0.6 kg, -2.6 kg, and -3.0 kg in the glimepiride, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The difference from glimepiride (95% CI) for ertugliflozin 5 mg was -3.2 kg (-3.7, -2.7) and for ertugliflozin 15 mg was -3.6 kg (-4.1, -3.1).
In Combination with Sitagliptin versus Ertugliflozin Alone and Sitagliptin Alone, as Add-on to Metformin
- A total of 1,233 patients with type 2 diabetes mellitus with inadequate glycemic control (HbA1c between 7.5% and 11%) on metformin monotherapy (≥1,500 mg/day for ≥8 weeks) participated in a randomized, double-blind, 26-week, active controlled study (NCT NCT02099110) to evaluate the efficacy and safety of ertugliflozin 5 mg or 15 mg in combination with sitagliptin 100 mg compared to the individual components. Patients were randomized to one of five treatment arms: ertugliflozin 5 mg, ertugliflozin 15 mg, sitagliptin 100 mg, ertugliflozin 5 mg + sitagliptin 100 mg, or ertugliflozin 15 mg + sitagliptin 100 mg.
- At Week 26, ertugliflozin 5 mg or 15 mg + sitagliptin 100 mg provided statistically significantly greater reductions in HbA1c compared to ertugliflozin (5 mg or 15 mg) alone or sitagliptin 100 mg alone. The mean change from baseline in HbA1c was -1.4% for ertugliflozin 5 mg or 15 mg + sitagliptin 100 mg versus -1.0%, for ertugliflozin 5 mg, ertugliflozin 15 mg, or sitagliptin 100 mg, respectively. More patients receiving ertugliflozin 5 mg or 15 mg + sitagliptin 100 mg achieved an HbA1c <7% (53.3% and 50.9%, for ertugliflozin 5 mg or 15 mg, respectively, + sitagliptin 100 mg) compared to the individual components (29.3%, 33.7%, and 38.5% for ertugliflozin 5 mg, ertugliflozin 15 mg, or sitagliptin 100 mg, respectively).
Add-on Combination Therapy with Metformin and Sitagliptin
- A total of 463 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) on metformin (≥1,500 mg/day for ≥8 weeks) and sitagliptin 100 mg once daily participated in a randomized, double-blind, multi-center, 26-week, placebo-controlled study (NCT02036515) to evaluate the efficacy and safety of ertugliflozin. Patients entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, ertugliflozin 5 mg, or ertugliflozin 15 mg.
- At Week 26, treatment with ertugliflozin at 5 mg or 15 mg daily provided statistically significant reductions in HbA1c. Ertugliflozin also resulted in a higher proportion of patients achieving an HbA1c <7% compared to placebo (see TABLE 7).

- The mean baseline body weight was 86.5 kg, 87.6 kg, and 86.6 kg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The mean changes from baseline to Week 26 were -1.0 kg, -3.0 kg, and -2.8 kg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The difference from placebo (95% CI) for ertugliflozin 5 mg was -1.9 kg (-2.6, -1.3) and for ertugliflozin 15 mg was -1.8 kg (-2.4, -1.2).
- The mean baseline systolic blood pressure was 130.2 mmHg, 132.1 mmHg, and 131.6 mmHg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The mean changes from baseline to Week 26 were -0.2 mmHg, -3.8 mmHg, and -4.5 mmHg in the placebo, ertugliflozin 5 mg, and ertugliflozin 15 mg groups, respectively. The difference from placebo (95% CI) for ertugliflozin 5 mg was -3.7 mmHg (-6.1, -1.2) and for ertugliflozin 15 mg was -4.3 mmHg (-6.7, -1.9).
Initial Combination Therapy with Sitagliptin
- A total of 291 patients with type 2 diabetes mellitus inadequately controlled (HbA1c between 8% and 10.5%) on diet and exercise participated in a randomized, double-blind, multi-center, placebo-controlled 26-week study (NCT02226003) to evaluate the efficacy and safety of ertugliflozin in combination with sitagliptin. These patients, who were not receiving any background antihyperglycemic treatment for ≥8 weeks, entered a 2-week, single-blind, placebo run-in period and were randomized to placebo, ertugliflozin 5 mg or ertugliflozin 15 mg in combination with sitagliptin (100 mg) once daily.
- At Week 26, treatment with ertugliflozin 5 mg and 15 mg in combination with sitagliptin at 100 mg daily provided statistically significant reductions in HbA1c compared to placebo. Ertugliflozin 5 mg and 15 mg in combination with sitagliptin at 100 mg daily also resulted in a higher proportion of patients achieving an HbA1c <7% and greater reductions in FPG compared with placebo.
Clinical Study of Ertugliflozin in Patients with Moderate Renal Impairment and Type 2 Diabetes Mellitus
- The efficacy of ertugliflozin was assessed in a multicenter, randomized, double-blind, placebo-controlled study (NCT01986855) of patients with type 2 diabetes mellitus and moderate renal impairment (468 patients with eGFR ≥30 to <60 mL/min/1.73 m2). In this study, 202 patients exposed to ertugliflozin (5 mg or 15 mg) had an eGFR between 45 and 60 mL/min/1.73 m2 and 111 patients exposed to ertugliflozin (5 mg or 15 mg) had an eGFR between 30 and 45 mL/min/1.73 m2. The mean duration of diabetes for the study population was approximately 14 years, and the majority of patients were receiving background insulin (55.9%) and/or sulfonylurea (40.3%) therapy. Approximately 50% had a history of cardiovascular disease or heart failure.
- Ertugliflozin did not show efficacy in this study. The HbA1c reductions from baseline to Week 26 were not significantly different between placebo and ertugliflozin 5 mg or 15 mg.
How Supplied
- Ertugliflozin tablets are available in the strengths listed below:
- 5 mg tablets, are pink, triangular-shaped, biconvex, with "701" debossed on one side and plain on the other side. They are supplied as follows:
- NDC 0006-5363-03 unit-of-use bottles of 30
- NDC 0006-5363-06 unit-of-use bottles of 90
- NDC 0006-5363-07 bulk bottles of 500
- 15 mg tablets, are red, triangular-shaped, biconvex, with "702" debossed on one side and plain on the other side. They are supplied as follows:
- NDC 0006-5364-03 unit-of-use bottles of 30
- NDC 0006-5364-06 unit-of-use bottles of 90
- NDC 0006-5364-07 bulk bottles of 500
Storage
- Store at 20°C -25°C (68°F -77°F), excursions permitted between 15°C -30°C (between 59°F -86°F). Protect from moisture. Store in a dry place.
Images
Drug Images
{{#ask: Page Name::Ertugliflozin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Ertugliflozin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Instructions
- Instruct patients to read the Medication Guide before starting ertugliflozin and to reread it each time the prescription is renewed.
- Inform patients of the potential risks and benefits of ertugliflozin and of alternative modes of therapy. Also inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. Advise patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery, as medication requirements may change.
- Instruct patients to take ertugliflozin only as prescribed. If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of ertugliflozin at the same time.
Hypoglycemia with Concomitant Use of Insulin and/or Insulin Secretagogue
- Inform patients that the incidence of hypoglycemia may increase when ertugliflozin is added to insulin and/or an insulin secretagogue and that a lower dose of insulin or insulin secretagogue may be required to reduce the risk of hypoglycemia
Hypotension
- Inform patients that symptomatic hypotension may occur with ertugliflozin and advise them to contact their doctor if they experience such symptoms. Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake.
Ketoacidosis
- Inform patients that ketoacidosis is a serious life-threatening condition. Cases of ketoacidosis have been reported during use of ertugliflozin. Instruct patients to check ketones (when possible) if symptoms consistent with ketoacidosis occur even if blood glucose is not elevated. If symptoms of ketoacidosis (including nausea, vomiting, abdominal pain, tiredness, and labored breathing) occur, instruct patients to discontinue ertugliflozin and seek medical advice immediately.
Acute Kidney Injury
- Inform patients that acute kidney injury has been reported during use of ertugliflozin. Advise patients to seek medical advice immediately if they have reduced oral intake (due to acute illness or fasting) or increased fluid losses (due to vomiting, diarrhea, or excessive heat exposure), as it may be appropriate to temporarily discontinue ertugliflozin use in those settings.
Monitoring of Renal Function
- Inform patients about the importance of regular testing of renal function when receiving treatment with ertugliflozin.
Serious Urinary Tract Infections
- Inform patients of the potential for urinary tract infections, which may be serious. Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur.
Amputation
- Inform patients of the potential for an increased risk of amputations. Counsel patients about the importance of routine preventative foot care. Instruct patients to monitor for new pain or tenderness, sores or ulcers, or infections involving the leg or foot and to seek medical advice immediately if such signs or symptoms develop.
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
- Inform female patients that vaginal yeast infections may occur and provide them with information on the signs and symptoms of vaginal yeast infection. Advise them of treatment options and when to seek medical advice.
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
- Inform male patients that yeast infections of the penis (e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males. Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). Advise them of treatment options and when to seek medical advice.
Fetal Toxicity
- Advise pregnant patients of the potential risk to a fetus with treatment with ertugliflozin. Instruct patients to immediately inform their healthcare provider if pregnant or planning to become pregnant.
Lactation
- Advise patients that use of ertugliflozin is not recommended while breastfeeding.
Laboratory Tests
- Due to its mechanism of action, inform patients that their urine will test positive for glucose while taking ertugliflozin.

Precautions with Alcohol
Alcohol-Ertugliflozin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Steglatro
Look-Alike Drug Names
There is limited information regarding Ertugliflozin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.