Enasidenib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2], Anmol Pitliya, M.B.B.S. M.D.[3]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
DIFFERENTIATION SYNDROME
See full prescribing information for complete Boxed Warning.
*Patients treated with Enasidenib have experienced symptoms of differentiation syndrome, which can be fatal if not treated. Symptoms may include fever, dyspnea, acute respiratory distress, pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain or peripheral edema, lymphadenopathy, bone pain, and hepatic, renal, or multi-organ dysfunction. If differentiation syndrome is suspected, initiate corticosteroid therapy and hemodynamic monitoring until symptom resolution.
|
Overview
Enasidenib is a isocitrate dehydrogenase-2 inhibitor that is FDA approved for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation as detected by an FDA-approved test. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, vomiting, diarrhea, elevated bilirubin, and decreased appetite.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Enasidenib is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation as detected by an FDA-approved test.
Recommended Dosage:
- The recommended starting dose of Enasidenib is 100 mg taken orally once daily with or without food until disease progression or unacceptable toxicity. For patients without disease progression or unacceptable toxicity, treat for a minimum of 6 months to allow time for clinical response.
- Do not split or crush Enasidenib tablets. Administer Enasidenib tablets orally about the same time each day. If a dose of Enasidenib is vomited, missed, or not taken at the usual time, administer the dose as soon as possible on the same day, and return to the normal schedule the following day.
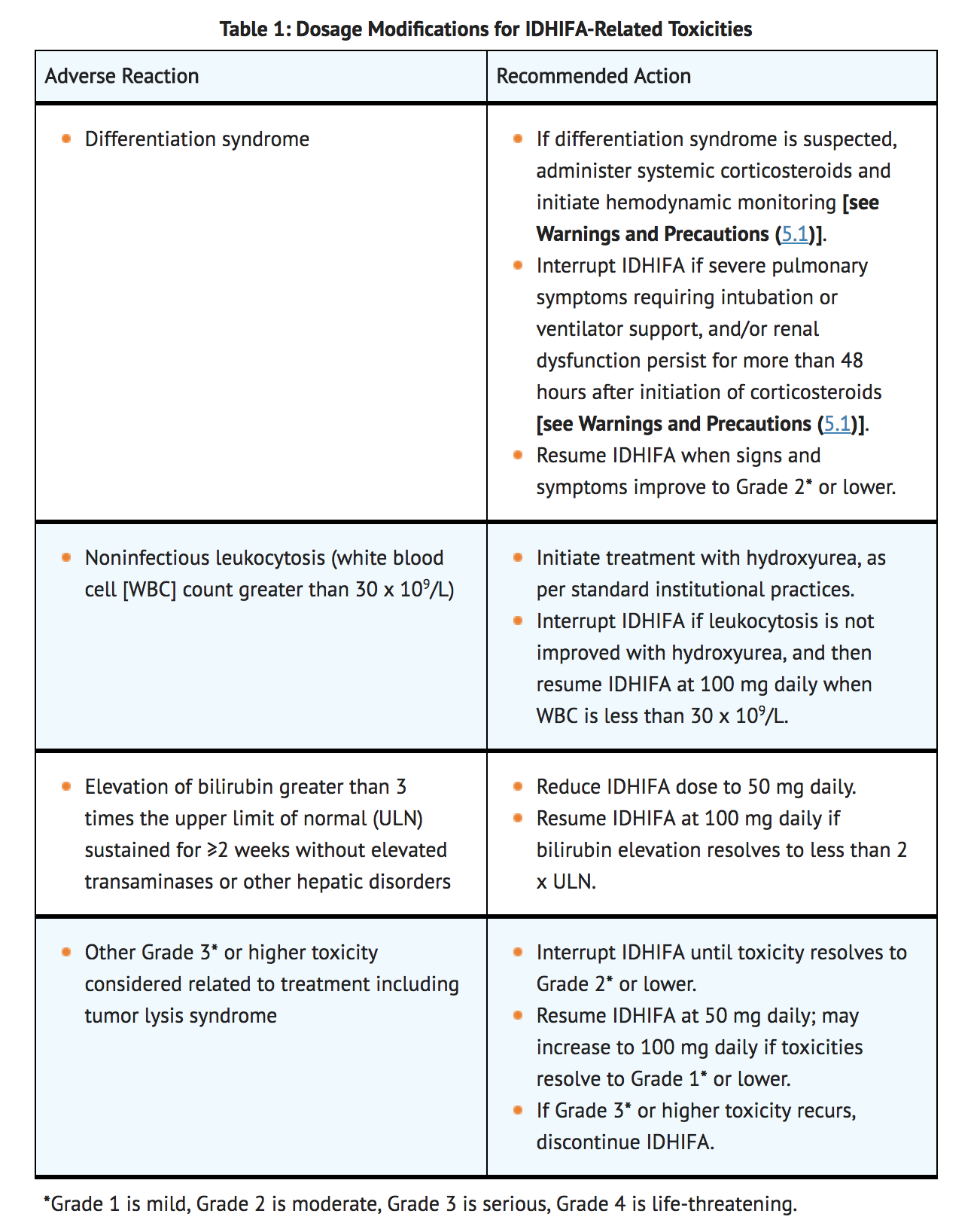
Monitoring and Dosage Modifications for Toxicities
- Assess blood counts and blood chemistries for leukocytosis and tumor lysis syndrome prior to the initiation of Enasidenib and monitor at a minimum of every 2 weeks for at least the first 3 months during treatment. Manage any abnormalities promptly.
- Interrupt dosing or reduce dose for toxicities. See TABLE 1 for dosage modification guidelines.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Enasidenib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Enasidenib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Enasidenib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Enasidenib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Enasidenib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None
Warnings
|
DIFFERENTIATION SYNDROME
See full prescribing information for complete Boxed Warning.
*Patients treated with Enasidenib have experienced symptoms of differentiation syndrome, which can be fatal if not treated. Symptoms may include fever, dyspnea, acute respiratory distress, pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain or peripheral edema, lymphadenopathy, bone pain, and hepatic, renal, or multi-organ dysfunction. If differentiation syndrome is suspected, initiate corticosteroid therapy and hemodynamic monitoring until symptom resolution.
|
Differentiation Syndrome
- In the clinical trial, 14% of patients treated with Enasidenib experienced differentiation syndrome, which may be life-threatening or fatal if not treated. Differentiation syndrome is associated with rapid proliferation and differentiation of myeloid cells. While there is no diagnostic test for differentiation syndrome, symptoms in patients treated with Enasidenib included acute respiratory distress represented by dyspnea and/or hypoxia (68%) and need for supplemental oxygen (76%); pulmonary infiltrates (73%) and pleural effusion (45%); renal impairment (70%); fever (36%); lymphadenopathy (33%); bone pain (27%); peripheral edema with rapid weight gain (21%); and pericardial effusion (18%). Hepatic, renal, and multi-organ dysfunction have also been observed. Differentiation syndrome has been observed with and without concomitant hyperleukocytosis, and as early as 10 days and at up to 5 months after Enasidenib initiation.
- If differentiation syndrome is suspected, initiate oral or intravenous corticosteroids (e.g., dexamethasone 10 mg every 12 hours) and hemodynamic monitoring until improvement. Taper corticosteroids only after resolution of symptoms. Symptoms of differentiation syndrome may recur with premature discontinuation of corticosteroid treatment. If severe pulmonary symptoms requiring intubation or ventilator support, and/or renal dysfunction persist for more than 48 hours after initiation of corticosteroids, interrupt Enasidenib until signs and symptoms are no longer severe. Hospitalization for close observation and monitoring of patients with pulmonary and/or renal manifestation is recommended.
Embryo-Fetal Toxicity
- Based on animal embryo-fetal toxicity studies, Enasidenib can cause embryo-fetal harm when administered to a pregnant woman. In animal embryo-fetal toxicity studies, Enasidenib caused embryo-fetal toxicities starting at 0.1 times the steady state clinical exposure based on the area under the concentration-time curve (AUC) at the recommended human dose. Advise females of reproductive potential to use effective contraception during treatment with Enasidenib and for at least 1 month after the last dose of Enasidenib. Advise males with female partners of reproductive potential to use effective contraception during treatment with Enasidenib and for at least 1 month after the last dose of Enasidenib. Pregnant women, patients becoming pregnant while receiving Enasidenib, or male patients with pregnant female partners should be apprised of the potential risk to the fetus.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety evaluation of single-agent Enasidenib is based on 214 patients with relapsed or refractory AML who were assigned to receive 100 mg daily [see Clinical Studies (14.1)]. The median duration of exposure to Enasidenib was 4.3 months (range 0.3 to 23.6). The 30-day and 60-day mortality rates observed with Enasidenib were 4.2% (9/214) and 11.7% (25/214), respectively.
- The most common adverse reactions (≥20%) of any grade were nausea, vomiting, diarrhea, elevated bilirubin and decreased appetite.
- Serious adverse reactions were reported in 77.1% of patients. The most frequent serious adverse reactions (≥2%) were leukocytosis (10%), diarrhea (6%), nausea (5%), vomiting (3%), decreased appetite (3%), tumor lysis syndrome (5%), and differentiation syndrome (8%). Differentiation syndrome events characterized as serious included pyrexia, renal failure acute, hypoxia, respiratory failure, and multi-organ failure.
- Overall, 92 of 214 patients (43%) required a dose interruption due to an adverse reaction; the most common adverse reactions leading to dose interruption were differentiation syndrome (4%) and leukocytosis (3%). Ten of 214 patients (5%) required a dose reduction due to an adverse reaction; no adverse reaction required dose reduction in more than 2 patients. Thirty-six of 214 patients (17%) permanently discontinued Enasidenib due to an adverse reaction; the most common adverse reaction leading to permanent discontinuation was leukocytosis (1%).
- Adverse reactions reported in the trial are shown in TABLE 2.

- Other clinically significant adverse reactions occurring in ≤10% of patients included:
- Respiratory, Thoracic, and Mediastinal Disorders: Pulmonary edema, acute respiratory distress syndrome.
- Changes in selected post-baseline laboratory values that were observed in patients with relapsed or refractory AML are shown in TABLE 3.

Elevated Bilirubin
- Enasidenib may interfere with bilirubin metabolism through inhibition of UGT1A1. Thirty-seven percent of patients (80/214) experienced total bilirubin elevations ≥2 x ULN at least one time. Of those patients who experienced total bilirubin elevations ≥2 x ULN, 35% had elevations within the first month of treatment, and 89% had no concomitant elevation of transaminases or other severe adverse events related to liver disorders. No patients required a dose reduction for hyperbilirubinemia; treatment was interrupted in 3.7% of patients, for a median of 6 days. Three patients (1.4%) discontinued Enasidenib permanently due to hyperbilirubinemia.
Non-infectious Leukocytosis
- Enasidenib can induce myeloid proliferation resulting in a rapid increase in white blood cell count.
Tumor Lysis Syndrome
- Enasidenib can induce myeloid proliferation resulting in a rapid reduction in tumor cells which may pose a risk for tumor lysis syndrome.
Postmarketing Experience
There is limited information regarding Enasidenib Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Enasidenib Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- Based on animal embryo-fetal toxicity studies, Enasidenib can cause fetal harm when administered to a pregnant woman. There are no available data on Enasidenib use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In animal embryo-fetal toxicity studies, oral administration of Enasidenib to pregnant rats and rabbits during organogenesis was associated with embryo-fetal mortality and alterations to growth starting at 0.1 times the steady state clinical exposure based on the AUC at the recommended human dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise the patient of the potential risk to a fetus.
- Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data (Animal)
- Enasidenib administered to pregnant rats at a dose of 30 mg/kg twice daily during organogenesis (gestation days 6-17) was associated with maternal toxicity and adverse embryo-fetal effects including post-implantation loss, resorptions, decreased viable fetuses, lower fetal birth weights, and skeletal variations. These effects occurred in rats at approximately 1.6 times the clinical exposure at the recommended human daily dose of 100 mg/day.
- In pregnant rabbits treated during organogenesis (gestation days 7-19), Enasidenib was maternally toxic at doses equal to 5 mg/kg/day or higher (exposure approximately 0.1 to 0.6 times the steady state clinical exposure at the recommended daily dose) and caused spontaneous abortions at 5 mg/kg/day (exposure approximately 0.1 times the steady state clinical exposure at the recommended daily dose).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Enasidenib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Enasidenib during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of Enasidenib or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for adverse reactions in breastfed infants, advise women not to breastfeed during treatment with Enasidenib and for at least 1 month after the last dose.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- No dosage adjustment is required for Enasidenib based on age. In the clinical study, 61% of 214 patients were aged 65 years or older, while 24% were older than 75 years. No overall differences in effectiveness or safety were observed between patients aged 65 years or older and younger patients.
Gender
There is no FDA guidance on the use of Enasidenib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Enasidenib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Enasidenib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Enasidenib in patients with hepatic impairment.
Females of Reproductive Potential and Males
Pregnancy Testing
- Based on animal embryo-fetal toxicity studies, Enasidenib can cause fetal harm when administered to a pregnant woman.
- Obtain a pregnancy test on females of reproductive potential prior to starting treatment with Enasidenib.
Contraception
Females
- Advise females of reproductive potential to avoid becoming pregnant while receiving Enasidenib. Advise females of reproductive potential to use effective contraception during treatment with Enasidenib and for at least 1 month after the last dose. Coadministration of Enasidenib may increase or decrease the concentrations of combined hormonal contraceptives. The clinical significance of this potential drug interaction is unknown at this time.
Males
- Advise males with female partners of reproductive potential to use effective contraception during treatment with Enasidenib and for at least 1 month after the last dose of Enasidenib.
Infertility
- Based on findings in animals, Enasidenib may impair fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible.
Immunocompromised Patients
There is no FDA guidance one the use of Enasidenib in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
- Administer at the same time each day.
- Do not split or crush tablets.
- Missed dose or vomiting: Administer as soon as possible on the same day and return to the normal schedule the following day.
Monitoring
- Screen for presence of IDH2 mutations prior to initiation of treatment.
- Disease response or stabilizations may indicate efficacy.
- Blood counts and blood chemistries: Prior to treatment and at least every 2 weeks for the first 3 months during treatment.
- Pregnancy test: Prior to treatment.
IV Compatibility
There is limited information regarding the compatibility of Enasidenib and IV administrations.
Overdosage
There is limited information regarding Enasidenib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Enasidenib
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Enasidenib is a small molecule inhibitor of the isocitrate dehydrogenase 2 (IDH2) enzyme. Enasidenib targets the mutant IDH2 variants R140Q, R172S, and R172K at approximately 40-fold lower concentrations than the wild-type enzyme in vitro. Inhibition of the mutant IDH2 enzyme by Enasidenib led to decreased 2-hydroxyglutarate (2-HG) levels and induced myeloid differentiation in vitro and in vivo in mouse xenograft models of IDH2 mutated AML. In blood samples from patients with AML with mutated IDH2, Enasidenib decreased 2-HG levels, reduced blast counts and increased percentages of mature myeloid cells.
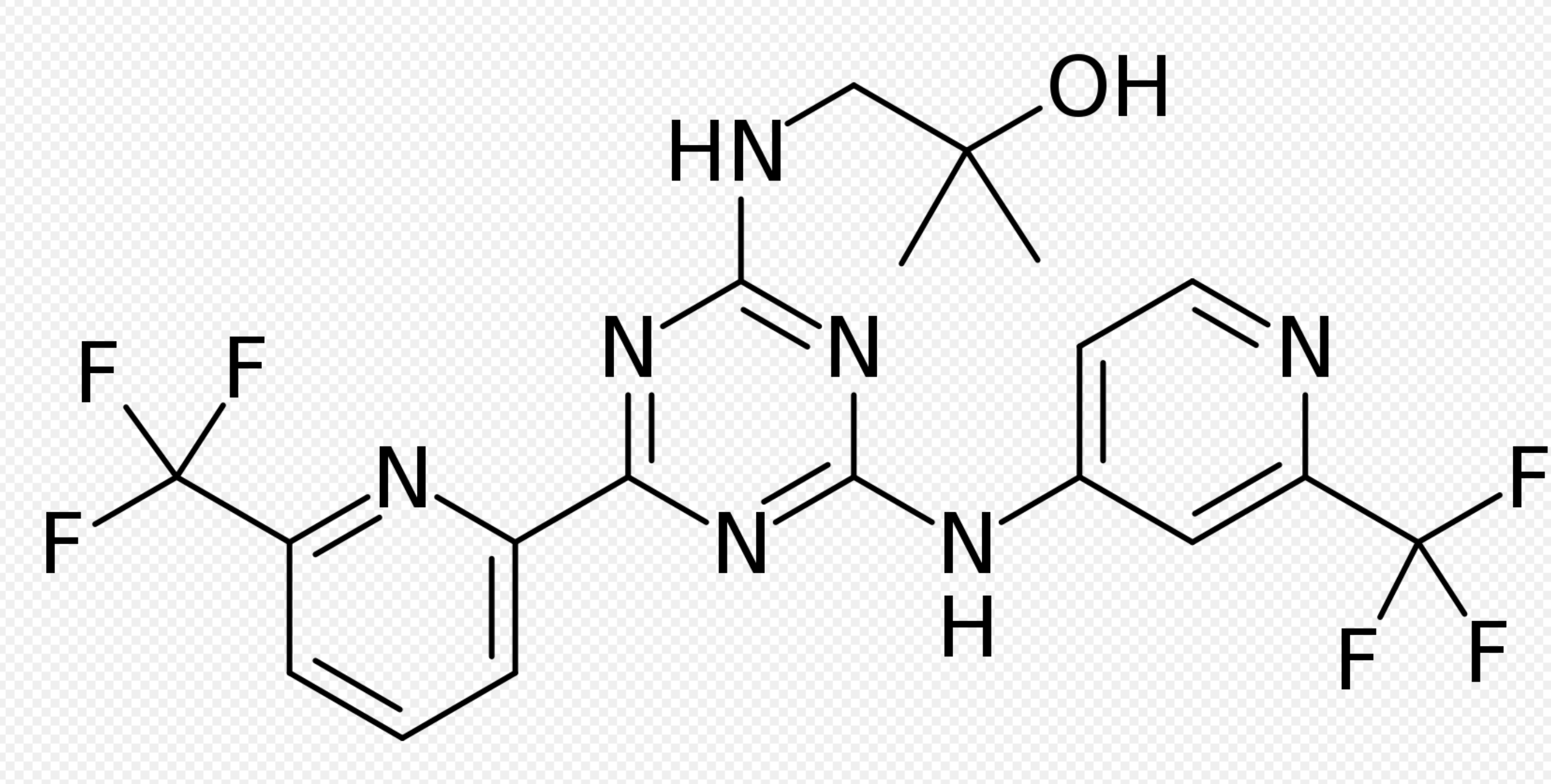
Structure

Pharmacodynamics
Cardiac Electrophysiology
- The potential for QTc prolongation with Enasidenib was evaluated in an open-label study in patients with advanced hematologic malignancies with an IDH2 mutation. Based on the QTc data for a single dose of 30 mg to 650 mg and multiple doses of 100 mg daily in the fasted state, no large mean changes in the QTc interval (>20 ms) were observed following treatment with Enasidenib.
Pharmacokinetics
- The peak plasma concentration (Cmax) is 1.3 mcg/mL [% coefficient of variation (CV%) 56.4] after a single dose of 100 mg, and 13 mcg/mL (CV% 46.3) at steady state for 100 mg daily. The area under concentration time curve (AUC) of Enasidenib increases in an approximately dose proportional manner from 50 mg (0.5 times approved recommended dosage) to 450 mg (4.5 times approved recommended dosage) daily dose. Steady-state plasma levels are reached within 29 days of once-daily dosing. Accumulation is approximately 10-fold when administered once daily.
Absorption
- The absolute bioavailability after 100 mg oral dose of Enasidenib is approximately 57%. After a single oral dose, the median time to Cmax (Tmax) is 4 hours.
=Distribution
- The mean volume of distribution (Vd) of Enasidenib is 55.8 L (CV% 29). Human plasma protein binding of Enasidenib is 98.5% and of its metabolite AGI-16903 is 96.6% in vitro.
- Enasidenib is not a substrate for P-glycoprotein or BCRP, while AGI-16903 is a substrate of both P-glycoprotein and BCRP. Enasidenib and AGI-16903 are not substrates of MRP2, OAT1, OAT3, OATP1B1, OATP1B3, and OCT2.
Elimination
- Enasidenib has a terminal half-life of 137 hours (CV% 41) and a mean total body clearance (CL/F) of 0.74 L/hour (CV% 71).
Metabolism
- Enasidenib accounted for 89% of the radioactivity in circulation and AGI-16903, the N-dealkylated metabolite, represented 10% of the circulating radioactivity.
- In vitro studies suggest that metabolism of Enasidenib is mediated by multiple CYP enzymes (e.g., CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4), and by multiple UGTs (e.g., UGT1A1, UGT1A3, UGT1A4, UGT1A9, UGT2B7, and UGT2B15). Further metabolism of the metabolite AGI-16903 is also mediated by multiple enzymes (e.g., CYP1A2, CYP2C19, CYP3A4, UGT1A1, UGT1A3, and UGT1A9).
Excretion
- Eighty-nine percent (89%) of Enasidenib is eliminated in feces and 11% in the urine. Excretion of unchanged Enasidenib accounts for 34% of the radiolabeled drug in the feces and 0.4% in the urine.
Specific Populations
- No clinically meaningful effect on the pharmacokinetics of Enasidenib was observed for the following covariates: age (19 years to 100 years), race (White, Black, or Asian), mild hepatic impairment [defined as total bilirubin ≤ upper limit of normal (ULN) and aspartate transaminase (AST) >ULN or total bilirubin 1 to 1.5 times ULN and any AST], renal impairment (defined as creatinine clearance ≥30 mL/min by Cockcroft-Gault formula), sex, body weight (39 kg to 136 kg), and body surface area.
Drug Interaction Studies
- In vitro studies suggest that Enasidenib inhibits the activity of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, and UGT1A1. Enasidenib inhibits P-gp, BCRP, OAT1, OATP1B1, OATP1B3, and OCT2, but not MRP2 or OAT3. Enasidenib induces CYP2B6 and CYP3A4.
- In vitro studies suggest that the metabolite AGI-16903 inhibits the activity of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6. AGI-16903 inhibits BCRP, OAT1, OAT3, OATP1B1, and OCT2, but not P-gp, MRP2, or OATP1B3.
- Coadministration of Enasidenib may increase or decrease the concentrations of combined hormonal contraceptives. The clinical significance of this potential drug interaction is unknown at this time.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been performed with Enasidenib.
- Enasidenib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay. Enasidenib was not clastogenic in an in vitro human lymphocyte chromosomal aberration assay, or in an in vivo rat bone marrow micronucleus assay.
- Fertility studies in animals have not been conducted with Enasidenib. In repeat-dose toxicity studies with twice daily oral administration of Enasidenib in rats up to 90-days in duration, changes were reported in male and female reproductive organs including seminiferous tubular degeneration, hypospermia, atrophy of the seminal vesicle and prostate, decreased corpora lutea and increased atretic follicles in the ovaries, and atrophy in the uterus.
Clinical Studies
Acute Myeloid Leukemia
- The efficacy of Enasidenib was evaluated in an open-label, single-arm, multicenter, two-cohort clinical trial (Study AG221-C-001, NCT01915498) of 199 adult patients with relapsed or refractory AML and an IDH2 mutation, who were assigned to receive 100 mg daily dose. Cohort 1 included 101 patients and Cohort 2 included 98 patients. IDH2 mutations were identified by a local diagnostic test and retrospectively confirmed by the Abbott RealTime™ IDH2 assay, or prospectively identified by the Abbott RealTime™ IDH2 assay, which is the FDA-approved test for selection of patients with AML for treatment with Enasidenib. Enasidenib was given orally at starting dose of 100 mg daily until disease progression or unacceptable toxicity. Dose reductions were allowed to manage adverse events.
- The baseline demographic and disease characteristics are shown in TABLE 4. The baseline demographics and disease characteristics were similar in both study cohorts.

- Efficacy was established on the basis of the rate of complete response (CR)/complete response with partial hematologic recovery (CRh), the duration of CR/CRh, and the rate of conversion from transfusion dependence to transfusion independence. The efficacy results are shown in TABLE 5 and were similar in both cohorts. The median follow-up was 6.6 months (range, 0.4 to 27.7 months). Similar CR/CRh rates were observed in patients with either R140 or R172 mutation.

- For patients who achieved a CR/CRh, the median time to first response was 1.9 months (range, 0.5 to 7.5 months) and the median time to best response of CR/CRh was 3.7 months (range, 0.6 to 11.2 months). Of the 46 patients who achieved a best response of CR/CRh, 39 (85%) did so within 6 months of initiating Enasidenib.
- Among the 157 patients who were dependent on red blood cell (RBC) and/or platelet transfusions at baseline, 53 (34%) became independent of RBC and platelet transfusions during any 56-day post baseline period. Of the 42 patients who were independent of both RBC and platelet transfusions at baseline, 32 (76%) remained transfusion independent during any 56-day post baseline period.
How Supplied
- 50-mg tablet: Pale yellow to yellow oval-shaped film-coated tablet debossed “ENA” on one side and “50” on the other side.
- 30-count bottles of 50-mg tablets with a desiccant canister (NDC 59572-705-30).
- 100-mg tablet: Pale yellow to yellow capsule-shaped film-coated tablet debossed “ENA” on one side and “100” on the other side.
- 30-count bottles of 100-mg tablets with a desiccant canister (NDC 59572-710-30).
Storage
- Store at 20°C-25°C (68°F-77°F); excursions permitted between 15°C-30°C (59°F-86°F). Keep the bottle tightly closed. Store in the original bottle (with a desiccant canister) to protect from moisture.
Images
Drug Images
{{#ask: Page Name::Enasidenib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Enasidenib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Differentiation Syndrome
- Advise patients on the risks of developing differentiation syndrome as early as 10 days and during the first 5 months on treatment. Ask patients to immediately report any symptoms suggestive of differentiation syndrome, such as fever, cough or difficulty breathing, bone pain, rapid weight gain or swelling of their arms or legs, to their healthcare provider for further evaluation.
Tumor Lysis Syndrome
- Advise patients on the risks of developing tumor lysis syndrome. Advise patients on the importance of maintaining high fluid intake, and the need for frequent monitoring of blood chemistry values.
Gastrointestinal Adverse Reactions
- Advise patients on risk of experiencing gastrointestinal reactions such as diarrhea, nausea, vomiting, decreased appetite, and changes in their sense of taste. Ask patients to report these events to their healthcare provider, and advise patients how to manage them.
Elevated Blood Bilirubin
- Inform patients that taking Enasidenib may cause elevated blood bilirubin, which is due to its mechanism of action, and not due to liver damage. Advise patients to report any changes to the color of their skin or the whites of their eyes to their healthcare provider for further evaluation.
Embryo-Fetal Toxicity and Use of Contraceptives
- Advise female patients with reproductive potential to use effective contraceptive methods while receiving Enasidenib and to avoid pregnancy while on treatment and for 1 month after completion of treatment. Advise patients to notify their healthcare provider immediately in the event of a pregnancy or if pregnancy is suspected during Enasidenib treatment. Advise males with female partners of reproductive potential to use effective contraception during treatment with Enasidenib and for at least 1 month after the last dose of Enasidenib. Coadministration of Enasidenib may increase or decrease the concentrations of combined hormonal contraceptives. The clinical significance of this potential drug interaction is unknown at this time.
Lactation
- Advise women not to breastfeed during treatment with Enasidenib and for at least 1 month after the final dose.
Dosing and Storage Instructions
- Advise patients not to chew or split the tablets but swallow whole with a cup of water.
- Instruct patients that if they miss a dose or vomit after a dose of Enasidenib, to take it as soon as possible on the same day and return to normal schedule the following day. Warn patients not to take 2 doses to make up for the missed dose.
- Keep Enasidenib in the original container. Keep the container tightly closed with desiccant canister inside to protect the tablets from moisture.

Precautions with Alcohol
Alcohol-Enasidenib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Idhifa
Look-Alike Drug Names
There is limited information regarding Enasidenib Look-Alike Drug Names in the drug label.
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.