Ecothiopate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Phospholine |
| Routes of administration | Topical (eye drops) |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
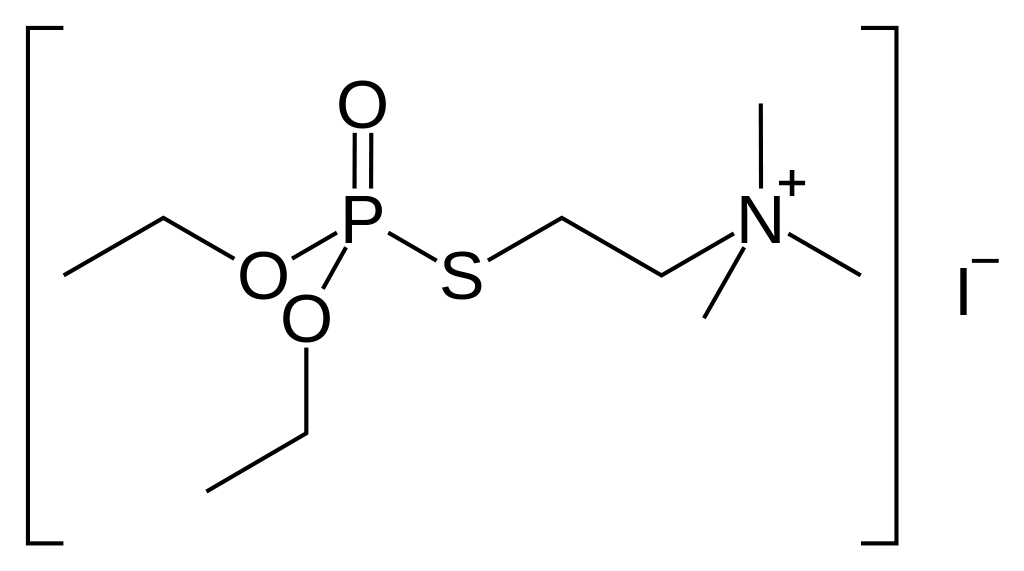
| Formula | C9H23INO3PS |
| Molar mass | 383.228 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Ecothiopate |
|
Articles |
|---|
|
Most recent articles on Ecothiopate Most cited articles on Ecothiopate |
|
Media |
|
Powerpoint slides on Ecothiopate |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ecothiopate at Clinical Trials.gov Clinical Trials on Ecothiopate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ecothiopate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Ecothiopate Discussion groups on Ecothiopate Patient Handouts on Ecothiopate Directions to Hospitals Treating Ecothiopate Risk calculators and risk factors for Ecothiopate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ecothiopate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Echothiophate (Phospholine) is a parasympathomimetic and a phosphorothioate. It is an irreversible acetylcholinesterase inhibitor.[1]
Uses
It is used as an ocular antihypertensive in the treatment of chronic glaucoma and, in some cases, accommodative esotropia. It is available under several trade names such as Phospholine Iodide (Wyeth-Ayerst).
Echothiophate binds irreversibly to cholinesterase. Because of the very slow rate at which echothiophate is hydrolyzed by cholinesterase, its effects can last a week or more. Adverse effects include muscle spasm and other systemic effects.
Mechanism of action
It covalently binds by its phosphate group to serine group at the active site of the cholinesterase. Once bound, the enzyme is permanently inactive and the cell has to make new enzymes.
Shortage
Wyeth Pharmaceuticals stopped manufacturing echothiophate iodide in the US in 2003. After contacting the American Academy of Ophthalmology (AAO), Wyeth rescinded their decision and, according to AAO public relations representative Michelle Stephens, the AAO and Wyeth were in talks for about a year about manufacturing it. [2]
In the meantime, a worldwide shortage of the drug has occurred.
Chemistry
Echothiophate is made by reacting diethylchlorophosphoric acid with 2-dimethylaminoethylmercaptan, giving S-(2-dimethylaminoethyl)-O,O-diethylthiophosphate, which is alkylated by methyl iodide, forming echothiophate.[3]
References
- ↑ Gabelt BT, Hennes EA, Seeman JL, Tian B, Kaufman PL (August 2004). "H-7 effect on outflow facility after trabecular obstruction following long-term echothiophate treatment in monkeys". Invest. Ophthalmol. Vis. Sci. 45 (8): 2732–6. doi:10.1167/iovs.04-0083. PMID 15277498.
- ↑ Eurotimes article: "Echothiophate iodide shortage leaves US specialists struggling to find alternative for acute cases"
- ↑ H.M. Fitch, Template:US Patent (1959)
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Quaternary ammonium compounds
- Iodides
- Drug
