Diclofenac (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Diclofenac (ophthalmic) is a nonsteroidal, anti-inflammatory drug that is FDA approved for the treatment of postoperative inflammation in patients who have undergone cataract extraction. Common adverse reactions include burning sensation in eye, keratitis, lacrimation and lacrimal drainage.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Diclofenac Sodium Ophthalmic Solution, 0.1% is indicated for the treatment of postoperative inflammation in patients who have undergone cataract extraction.
Dosage
- One drop of Diclofenac Sodium Ophthalmic Solution, 0.1% should be applied to the affected eye four times daily beginning 24 hours after cataract surgery and continuing throughout the first 2 weeks of the postoperative period.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Diclofenac (ophthalmic) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Diclofenac (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Diclofenac (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Diclofenac (ophthalmic) in pediatric patients.
Contraindications
- Diclofenac Sodium Ophthalmic Solution, 0.1% is contraindicated in patients who are hypersensitive to any component of the medication.
Warnings
- With some nonsteroidal anti-inflammatory drugs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
- There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other non-steroidal anti-inflammatory agents. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
Precations
General
- It is recommended that Diclofenac Sodium Ophthalmic Solution, 0.1%, like other NSAIDs, be used with caution in surgical patients with known bleeding tendencies or who are receiving other medications that may prolong bleeding time.
- Diclofenac Sodium Ophthalmic Solution, 0.1% may slow or delay healing.
- Results from clinical studies indicate that Diclofenac Sodium Ophthalmic Solution, 0.1% has no significant effect upon intraocular pressure, however, elevations in intraocular pressure may occur following cataract surgery.
Adverse Reactions
Clinical Trials Experience
Ocular
- Transient burning and stinging was reported in 15% of patients with the use of topical diclofenac sodium ophthalmic solution, 0.1%. In cataract studies, keratitis occurred in 28% of patients receiving diclofenac sodium ophthalmic solution, 0.1%; however, most of the cases of keratitis occurred prior to drug therapy. Elevated intraocular pressure was reported in 15% of patients receiving diclofenac sodium ophthalmic solution, 0.1%. The following adverse reactions were reported in approximately 5% or less of the patients: abnormal vision, anterior chamber reaction, blurred vision, conjunctivitis, corneal deposits, corneal edema, corneal lesions, corneal opacity, discharge, injection, iritis, irritation, itching and ocular allergy.
Systemic
- The following adverse reactions were reported in 3% or less of the patients: abdominal pain, asthenia, chills, dizziness, facial edema, fever, headache, insomnia, nausea, pain, rhinitis, viral infection, and vomiting.
Postmarketing Experience
- There is limited information regarding postmarketing experience
Drug Interactions
- There is limited information regarding Drug Interactions
Use in Specific Populations
Pregnancy
- Reproduction studies performed in mice at oral doses up to 5000 times (20 mg/kg/day) and in rats and rabbits at oral doses up to 2500 times (10 mg/kg/day) the human topical dose have revealed no evidence of teratogenicity due to diclofenac sodium, despite the induction of maternal toxicity and fetal toxicity. In rats, maternally toxic doses were associated with dystocia, prolonged gestation, reduced fetal weights and growth, and reduced fetal survival. Diclofenac sodium has been shown to cross the placental barrier in mice and rats.
- There are however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic effects
- Because of the known effects of prostaglandin biosynthesis-inhibiting drugs on the fetal cardiovascular system (closure of the ductus arteriosus), the use of Diclofenac Sodium Ophthalmic Solution, 0.1% during late pregnancy should be avoided.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diclofenac (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Diclofenac (ophthalmic) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Diclofenac (ophthalmic) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Diclofenac (ophthalmic) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Diclofenac (ophthalmic) in geriatric settings.
Gender
There is no FDA guidance on the use of Diclofenac (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diclofenac (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diclofenac (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Diclofenac (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diclofenac (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diclofenac (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic.
Monitoring
- There is limited information regarding drug monitoring.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- Overdosage will not ordinarily cause acute problems. If accidentally ingested, fluids should be taken to dilute the medication.
Pharmacology
Mechanism of Action
- Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action of diclofenac potassium, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
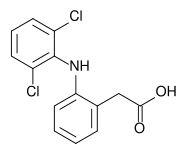
Structure
- Diclofenac Sodium Ophthalmic Solution, 0.1% is a sterile, topical, nonsteroidal, anti-inflammatory product for ophthalmic use. Diclofenac sodium is designated chemically as 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt, with an empirical formula of C14H10Cl2NO2Na. The structural formula of diclofenac sodium is:

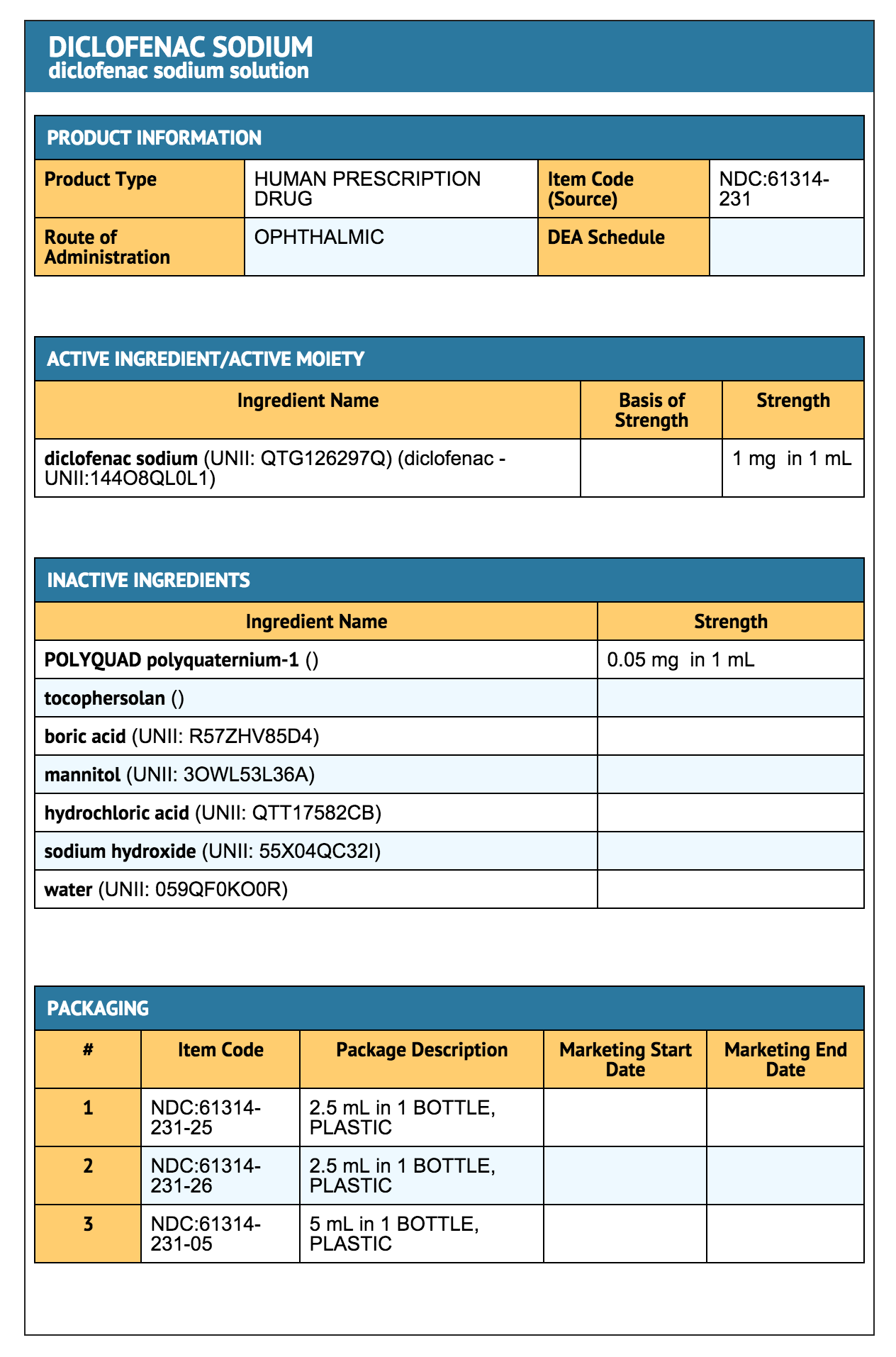
Diclofenac Sodium Ophthalmic Solution, 0.1% is available as a sterile solution which contains diclofenac sodium 0.1% (1 mg/mL).
- Inactive Ingredients: POLYQUAD® polyquaternium-1, 0.05 mg/mL, as preservative, tocophersolan, boric acid, mannitol, hydrochloric acid and/or sodium hydroxide (to adjust pH to 6.7) and purified water. DM-00
- Diclofenac sodium is a faintly yellow-white to light-beige, slightly hygroscopic crystalline powder. It is freely soluble in methanol, sparingly soluble in water, very slightly soluble in acetonitrile, and insoluble in chloroform and in 0.1N hydrochloric acid. Its molecular weight is 318.14. Diclofenac Sodium Ophthalmic Solution, 0.1% is an iso-osmotic solution with an osmolality of about 300 mOsmol/1000g.
Pharmacodynamics
- Diclofenac sodium is one of a series of phenylacetic acids that have demonstrated anti-inflammatory and analgesic properties in pharmacological studies. It is thought to inhibit the enzyme cyclooxygenase, which is essential in the biosynthesis of prostaglandins.
Pharmacokinetics
- Results from a bioavailability study of another formulation of diclofenac sodium ophthalmic solution established that plasma levels of diclofenac following ocular instillation of two drops of diclofenac sodium ophthalmic solution, 0.1% to each eye were below the limit of quantitation (10 ng/mL) over a 4-hour period. This study suggests that limited, if any, systemic absorption occurs with diclofenac sodium ophthalmic solution, 0.1%.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long term carcinogenicity studies in rats given oral diclofenac sodium up to 2 mg/kg/day (approximately the human oral dose) have revealed no significant increases in tumor incidence. There was a slight increase in benign rat mammary fibroadenomas in mid-dose females (high dose females had excessive mortality) but the increase was not significant for this common rat tumor. A 2-year carcinogenicity study conducted in mice employing oral diclofenac sodium up to 2 mg/kg/day did not reveal any oncogenic potential. Diclofenac sodium did not show mutagenic potential in various mutagenicity studies including the Ames test. Diclofenac sodium administered to male and female rats at 4 mg/kg/day did not affect fertility.
Clinical Studies
Postoperative Anti-Inflammatory Effects
- In a clinical therapeutic equivalence study, Diclofenac Sodium Ophthalmic Solution, 0.1%, was found to be therapeutically equivalent to VOLTAREN OPHTHALMIC® (diclofenac sodium ophthalmic solution) [CIBA VISION OPHTHALMICS®] in the treatment of signs and symptoms of inflammation resulting from cataract surgery.
How Supplied
- Diclofenac Sodium Ophthalmic Solution, 0.1% is supplied in opaque, plastic DROP-TAINER® dispenser in the following sizes:
- 2.5 mL: NDC 61314-231-26
- 5.0 mL: NDC 61314-231-05
Storage
- Store between 59° -86° F (15° -30° C). Protect from light. Dispense in original, unopened container only.
Images
Drug Images
{{#ask: Page Name::Diclofenac (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Diclofenac (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- There is limited information regarding Patient Counseling Information.
Precautions with Alcohol
Alcohol-Diclofenac (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DICLOFENAC SODIUM ®[1]
Look-Alike Drug Names
- There is limited information regarding Look-Alike Drug Names.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.