Bictegravir / emtricitabine / tenofovir alafenamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2], Anmol Pitliya, M.B.B.S. M.D.[3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
|
Overview
Bictegravir / emtricitabine / tenofovir alafenamide is a three-drug combination of bictegravir (BIC), a human immunodeficiency virus type 1 (HIV-1) integrase strand transfer inhibitor (INSTI), and emtricitabine (FTC) and tenofovir alafenamide (TAF), both HIV-1 nucleoside analog reverse transcriptase inhibitors (NRTIs) that is FDA approved for the treatment of HIV-1 infection in adults who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 3 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of bictegravir /emtricitabine /tenofovir alafenamide. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, nausea, and headache..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Bictegravir /emtricitabine /tenofovir alafenamide is indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults who have no antiretroviral treatment history or to replace the current antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 3 months with no history of treatment failure and no known substitutions associated with resistance to the individual components of bictegravir /emtricitabine /tenofovir alafenamide.
Recommended Dosage:
- Bictegravir /emtricitabine /tenofovir alafenamide is a three-drug fixed dose combination product containing 50 mg of bictegravir (BIC), 200 mg of emtricitabine (FTC), and 25 mg of tenofovir alafenamide (TAF). The recommended dosage of bictegravir /emtricitabine /tenofovir alafenamide is one tablet taken orally once daily with or without food.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding bictegravir/ emtricitabine/ tenofovir alafenamide Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding bictegravir/ emtricitabine/ tenofovir alafenamide Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Bictegravir / emtricitabine / tenofovir alafenamide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding bictegravir/ emtricitabine/ tenofovir alafenamide Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding bictegravir/ emtricitabine/ tenofovir alafenamide Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Bictegravir /emtricitabine /tenofovir alafenamide is contraindicated to be co-administered with:
- Dofetilide due to the potential for increased dofetilide plasma concentrations and associated serious and/or life-threatening events.
- Rifampin due to decreased BIC plasma concentrations, which may result in the loss of therapeutic effect and development of resistance to bictegravir /emtricitabine /tenofovir alafenamide.
Warnings
|
POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
|
Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
- Patients with HIV-1 should be tested for the presence of chronic hepatitis B virus (HBV) infection before or when initiating antiretroviral therapy.
- Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing FTC and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of bictegravir /emtricitabine /tenofovir alafenamide. Patients coinfected with HIV-1 and HBV who discontinue bictegravir /emtricitabine /tenofovir alafenamide should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since post-treatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
- The concomitant use of bictegravir /emtricitabine /tenofovir alafenamide with certain other drugs may result in known or potentially significant drug interactions, some of which may lead to:
- Loss of therapeutic effect of bictegravir /emtricitabine /tenofovir alafenamide and possible development of resistance.
- Possible clinically significant adverse reactions from greater exposures of concomitant drugs.
- See TABLE 3 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during bictegravir /emtricitabine /tenofovir alafenamide therapy; review concomitant medications during bictegravir /emtricitabine /tenofovir alafenamide therapy; and monitor for the adverse reactions associated with the concomitant drugs.
Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections [such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis], which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
New Onset or Worsening Renal Impairment
- Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of tenofovir prodrugs in both animal toxicology studies and human trials. In clinical trials of bictegravir /emtricitabine /tenofovir alafenamide, there have been no cases of Fanconi syndrome or Proximal Renal Tubulopathy (PRT). In clinical trials of bictegravir /emtricitabine /tenofovir alafenamide in subjects with no antiretroviral treatment history with eGFRs greater than 30 mL per minute, and in virologically suppressed subjects switched to bictegravir /emtricitabine /tenofovir alafenamide with eGFRs greater than 50 mL per minute, renal serious adverse events were encountered in less than 1% of subjects treated with bictegravir /emtricitabine /tenofovir alafenamide through Week 48. Bictegravir /emtricitabine /tenofovir alafenamide is not recommended in patients with estimated creatinine clearance below 30 mL per minute.
- Patients taking tenofovir prodrugs who have impaired renal function and those taking nephrotoxic agents including non-steroidal anti-inflammatory drugs are at increased risk of developing renal-related adverse reactions.
- Prior to or when initiating bictegravir /emtricitabine /tenofovir alafenamide, and during treatment with bictegravir /emtricitabine /tenofovir alafenamide, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients as clinically appropriate. In patients with chronic kidney disease, also assess serum phosphorus. Discontinue bictegravir /emtricitabine /tenofovir alafenamide in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome.
Lactic Acidosis/Severe Hepatomegaly with Steatosis
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including emtricitabine, a component of bictegravir /emtricitabine /tenofovir alafenamide, and tenofovir DF, another prodrug of tenofovir, alone or in combination with other antiretrovirals. Treatment with bictegravir /emtricitabine /tenofovir alafenamide should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Adults with No Antiretroviral Treatment History
- The primary safety assessment of bictegravir /emtricitabine /tenofovir alafenamide was based on Week 48 data from two randomized, double-blind, active-controlled trials, Trial 1489 and Trial 1490, that enrolled 1274 HIV-1 infected adult subjects with no antiretroviral treatment history. A total of 634 subjects received one tablet of bictegravir /emtricitabine /tenofovir alafenamide once daily.
- The most common adverse reactions (all Grades) reported in at least 5% of subjects in the bictegravir /emtricitabine /tenofovir alafenamide group in either Trial 1489 or Trial 1490 were diarrhea, nausea, and headache. The proportion of subjects who discontinued treatment with bictegravir /emtricitabine /tenofovir alafenamide, abacavir [ABC]/dolutegravir [DTG]/ lamivudine [3TC]), or DTG + FTC/TAF, due to adverse events, regardless of severity, was 1%, 1%, and <1%, respectively. Table 1 displays the frequency of adverse reactions (all Grades) greater than or equal to 2% in the bictegravir /emtricitabine /tenofovir alafenamide group.

- Additional adverse reactions (all Grades) occurring in less than 2% of subjects administered bictegravir /emtricitabine /tenofovir alafenamide in Trials 1489 and 1490 included vomiting, flatulence, dyspepsia, abdominal pain, rash, and depression.
- Suicidal ideation, suicide attempt, and depression suicidal occurred in <1% of subjects administered bictegravir /emtricitabine /tenofovir alafenamide; all events were serious and primarily occurred in subjects with a preexisting history of depression, prior suicide attempt or psychiatric illness.
- The majority (87%) of adverse events associated with bictegravir /emtricitabine /tenofovir alafenamide were Grade 1.
Clinical Trials in Virologically Suppressed Adults
- The safety of bictegravir /emtricitabine /tenofovir alafenamide in virologically-suppressed adults was based on Week 48 data from 282 subjects in a randomized, double-blind, active-controlled trial (Trial 1844) in which virologically-suppressed subjects were switched from either DTG + ABC/3TC or ABC/DTG/3TC to bictegravir /emtricitabine /tenofovir alafenamide; and Week 48 data from 290 subjects in an open-label, active-controlled trial in which virologically-suppressed subjects were switched from a regimen containing atazanavir (ATV) (given with cobicistat or ritonavir) or darunavir (DRV) (given with cobicistat or ritonavir) plus either FTC/TDF or ABC/3TC, to bictegravir /emtricitabine /tenofovir alafenamide (Trial 1878). Overall, the safety profile in virologically suppressed adult subjects in Trials 1844 and 1878 was similar to that in subjects with no antiretroviral treatment history.
Laboratory Abnormalities
- The frequency of laboratory abnormalities (Grades 3–4) occurring in at least 2% of subjects receiving bictegravir /emtricitabine /tenofovir alafenamide in Trials 1489 and 1490 are presented in Table 2.

- Changes in Serum Creatinine: BIC has been shown to increase serum creatinine due to inhibition of tubular secretion of creatinine without affecting renal glomerular function. Increases in serum creatinine occurred by Week 4 of treatment and remained stable through Week 48. In Trials 1489 and 1490, median (Q1, Q3) serum creatinine increased by 0.10 (0.03, 0.17) mg per dL from baseline to Week 48 in the bictegravir /emtricitabine /tenofovir alafenamide group and was similar to the comparator groups who received ABC/DTG/3TC, or DTG + FTC/TAF. There were no discontinuations due to renal adverse events through Week 48 in bictegravir /emtricitabine /tenofovir alafenamide clinical trials.
- Changes in Bilirubin: In Trials 1489 and 1490, total bilirubin increases were observed in 12% of subjects administered bictegravir /emtricitabine /tenofovir alafenamide through Week 48. Increases were primarily Grade 1 (1.0 to 1.5 × ULN) (9%) and Grade 2 (1.5 to 2.5 × ULN) (3%). Graded bilirubin increases in the ABC/DTG/3TC, and DTG + FTC/TAF groups, were 4% and 6%, respectively. Increases were primarily Grade 1 (3% ABC/DTG/3TC and 5% DTG + FTC/TAF) or Grade 2 (1% ABC/DTG/3TC and 1% DTG + FTC/TAF). There were no discontinuations due to hepatic adverse events through Week 48 in bictegravir /emtricitabine /tenofovir alafenamide clinical studies.
Postmarketing Experience
There is limited information regarding Bictegravir / emtricitabine / tenofovir alafenamide Postmarketing Experience in the drug label.
Drug Interactions
- Other Antiretroviral Medications
- Potential for bictegravir /emtricitabine /tenofovir alafenamide to Affect Other Drugs
- Potential Effect of Other Drugs on One or More Components of bictegravir /emtricitabine /tenofovir alafenamide
- Drugs Affecting Renal Function
- Established and Potentially Significant Drug Interactions
- Drugs without Clinically Significant Interactions with bictegravir /emtricitabine /tenofovir alafenamide
Other Antiretroviral Medications
- Because bictegravir /emtricitabine /tenofovir alafenamide is a complete regimen, coadministration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended. Comprehensive information regarding potential drug-drug interactions with other antiretroviral medications is not provided because the safety and efficacy of concomitant HIV-1 antiretroviral therapy is unknown.
Potential for Bictegravir /Emtricitabine /Tenofovir Alafenamide to Affect Other Drugs
- BIC inhibits organic cation transporter 2 (OCT2) and multidrug and toxin extrusion transporter 1 (MATE1) in vitro. Coadministration of bictegravir /emtricitabine /tenofovir alafenamide with drugs that are substrates of OCT2 and MATE1 (e.g., dofetilide) may increase their plasma concentrations (see TABLE 3).
Potential Effect of Other Drugs on One or More Components of Bictegravir /Emtricitabine /Tenofovir Alafenamide
- BIC is a substrate of CYP3A and UGT1A1. A drug that is a strong inducer of CYP3A and also an inducer of UGT1A1 can substantially decrease the plasma concentrations of BIC which may lead to loss of therapeutic effect of bictegravir /emtricitabine /tenofovir alafenamide and development of resistance.
Drugs Affecting Renal Function
- Because FTC and tenofovir are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion, coadministration of bictegravir /emtricitabine /tenofovir alafenamide with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of FTC, tenofovir, and other renally eliminated drugs and this may increase the risk of adverse reactions. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs.
Established and Potentially Significant Drug Interactions
- Table 3 provides a listing of established or potentially clinically significant drug interactions with recommended prevention or management strategies. The drug interactions described are based on studies conducted with either bictegravir /emtricitabine /tenofovir alafenamide, the components of bictegravir /emtricitabine /tenofovir alafenamide (BIC, FTC, and TAF) as individual agents, or are drug interactions that may occur with bictegravir /emtricitabine /tenofovir alafenamide.

Drugs without Clinically Significant Interactions with Bictegravir /Emtricitabine /Tenofovir Alafenamide
- Based on drug interaction studies conducted with bictegravir /emtricitabine /tenofovir alafenamide or the components of bictegravir /emtricitabine /tenofovir alafenamide, no clinically significant drug interactions have been observed when bictegravir /emtricitabine /tenofovir alafenamide is combined with the following drugs: ethinyl estradiol, ledipasvir/sofosbuvir, midazolam, norgestimate, sertraline, sofosbuvir, sofosbuvir/velpatasvir, and sofosbuvir/velpatasvir/voxilaprevir.
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
- There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to bictegravir /emtricitabine /tenofovir alafenamide during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
- There are insufficient human data on the use of bictegravir /emtricitabine /tenofovir alafenamide during pregnancy to inform a drug-associated risk of birth defects and miscarriage. Bictegravir (BIC) and tenofovir alafenamide (TAF) use in women during pregnancy has not been evaluated; however, emtricitabine (FTC) use during pregnancy has been evaluated in a limited number of women reported to the APR. Available data from the APR show no difference in the overall risk of major birth defects for FTC compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP). The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in the clinically recognized pregnancies in the U.S. general population is 15–20%. Methodological limitations of the APR include the use of MACDP as the external comparator group. The MACDP population is not disease-specific, evaluates women and infants from a limited geographic area, and does not include outcomes for births that occurred at less than 20 weeks gestation.
- In animal reproduction studies, no evidence of adverse developmental outcomes was observed with the components of bictegravir /emtricitabine /tenofovir alafenamide at exposures that were either not maternally toxic (rabbits) or greater than (rats and mice) those in humans at the recommended human dose (RHD). During organogenesis, systemic exposures (AUC) to BIC were approximately 36 (rats) and 0.6 times (rabbits), to FTC were approximately 60 (mice) and 108 times (rabbits), and to TAF were approximately 2 (rats) and 78 times (rabbits) the exposure at the RHD of bictegravir /emtricitabine /tenofovir alafenamide. In rat pre/postnatal development studies, maternal systemic exposures (AUC) were 30 times (BIC), 60 times (FTC), and 19 times (TDF) the exposures of each component in humans at the RHD.
Data (Human)
- Emtricitabine: Based on prospective reports to the APR of 3,406 exposures to FTC-containing regimens during pregnancy resulting in live births (including 2,326 exposed in the first trimester and 1,080 exposed in the second/third trimester), there was no difference between FTC and overall birth defects compared with the background birth defect rate of 2.7% in the U.S. reference population of the MACDP. The prevalence of birth defects in live births was 2.3% (95% CI: 1.7% to 3.0%) with first trimester exposure to FTC-containing regimens and 2.0% (95% CI: 1.3% to 3.1%) with the second/third trimester exposure to FTC-containing regimens.
Data (Animal)
- Bictegravir: BIC was administered orally to pregnant rats (5, 30, or 300 mg/kg/day) and rabbits (100, 300, or 1000 mg/kg/day) on gestation days 7 through 17, and 7 through 19, respectively. No adverse embryo-fetal effects were observed in rats and rabbits at BIC exposures (AUC) of up to approximately 36 (rats) and 0.6 (rabbits) times the exposure in humans at the RHD of bictegravir /emtricitabine /tenofovir alafenamide. Spontaneous abortion, increased clinical signs [fecal changes, thin body, and cold-to-touch], and decreased body weight were observed at a maternally toxic dose in rabbits (1000 mg/kg/day; approximately 1.4 times higher than human exposure at the RHD).
- In a pre/postnatal development study, BIC was administered orally to pregnant rats (up to 300 mg/kg/day) from gestation days 6 to lactation/post-partum day 24. No significant adverse effects were observed in the offspring exposed daily from before birth (in utero) through lactation at maternal and pup exposures (AUC) of approximately 30 and 11 times higher, respectively, than human exposures at the RHD.
- Emtricitabine: FTC was administered orally to pregnant mice (250, 500, or 1000 mg/kg/day) and rabbits (100, 300, or 1000 mg/kg/day) through organogenesis (on gestation days 6 through 15, and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with emtricitabine in mice at exposures approximately 60 times higher and in rabbits at approximately 108 times higher than human exposures at the RHD.
- In a pre/postnatal development study with FTC, mice were administered doses up to 1000 mg/kg/day; no significant adverse effects directly related to drug were observed in the offspring exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60 times higher than human exposures at the RHD.
- Tenofovir alafenamide: TAF was administered orally to pregnant rats (25, 100, or 250 mg/kg/day) and rabbits (10, 30, or 100 mg/kg/day) through organogenesis (on gestation days 6 through 17, and 7 through 20, respectively). No adverse embryo-fetal effects were observed in rats and rabbits at TAF exposures of approximately 2 (rats) and 78 (rabbits) times higher than the exposure in humans at the recommended daily dose of bictegravir /emtricitabine /tenofovir alafenamide. TAF is rapidly converted to tenofovir; the observed tenofovir exposure in rats and rabbits were 55 (rats) and 86 (rabbits) times higher than human tenofovir exposures at the RHD. Since TAF is rapidly converted to tenofovir and lower tenofovir exposures in rats and mice were observed after TAF administration compared to TDF administration, a pre/postnatal development study in rats was conducted only with TDF. Doses up to 600 mg/kg/day were administered through lactation; no adverse effects were observed in the offspring on gestation day 7 [and lactation day 20] at tenofovir exposures of approximately 12 [19] times higher than the exposures in humans at the RHD of bictegravir /emtricitabine /tenofovir alafenamide.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Bictegravir / emtricitabine / tenofovir alafenamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Bictegravir / emtricitabine / tenofovir alafenamide during labor and delivery.
Nursing Mothers
Risk Summary
- The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection.
- It is not known whether bictegravir /emtricitabine /tenofovir alafenamide or all of the components of bictegravir /emtricitabine /tenofovir alafenamide are present in human breast milk, affects human milk production, or has effects on the breastfed infant. Based on published data, FTC has been shown to be present in human breast milk. BIC was detected in the plasma of nursing rat pups likely due to the presence of BIC in milk, and tenofovir has been shown to be present in the milk of lactating rats and rhesus monkeys after administration of TDF. It is unknown if TAF is present in animal milk.
- Because of the potential for 1) HIV transmission (in HIV-negative infants); 2) developing viral resistance (in HIV-positive infants); and 3) adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are receiving bictegravir /emtricitabine /tenofovir alafenamide.
Data (Animal)
- Bictegravir: BIC was detected in the plasma of nursing rat pups in the pre/postnatal development study (post-natal day 10), likely due to the presence of BIC in milk.
- Tenofovir alafenamide: Studies in rats and monkeys have demonstrated that tenofovir is secreted in milk. Tenofovir was excreted into the milk of lactating rats following oral administration of TDF (up to 600 mg/kg/day) at up to approximately 24% of the median plasma concentration in the highest dosed animals at lactation day 11. Tenofovir was excreted into the milk of lactating monkeys following a single subcutaneous (30 mg/kg) dose of tenofovir at concentrations up to approximately 4% of plasma concentration, resulting in exposure (AUC) of approximately 20% of plasma exposure.
Pediatric Use
- Safety and effectiveness of bictegravir /emtricitabine /tenofovir alafenamide in pediatric patients less than 18 years of age have not been established.
Geriatic Use
- Clinical trials of bictegravir /emtricitabine /tenofovir alafenamide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Bictegravir / emtricitabine / tenofovir alafenamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Bictegravir / emtricitabine / tenofovir alafenamide with respect to specific racial populations.
Renal Impairment
- Bictegravir /emtricitabine /tenofovir alafenamide is not recommended in patients with severe renal impairment (estimated creatinine clearance (CLcr) below 30 mL per minute, estimated by Cockcroft-Gault (C-G). No dosage adjustment of bictegravir /emtricitabine /tenofovir alafenamide is recommended in patients with ClCr greater than or equal to 30 mL per minute.
Hepatic Impairment
- No dosage adjustment of bictegravir /emtricitabine /tenofovir alafenamide is recommended in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. Bictegravir /emtricitabine /tenofovir alafenamide has not been studied in patients with severe hepatic impairment (Child-Pugh Class C). Therefore, bictegravir /emtricitabine /tenofovir alafenamide is not recommended for use in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Bictegravir / emtricitabine / tenofovir alafenamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Bictegravir / emtricitabine / tenofovir alafenamide in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
- Administer with or without food.
Monitoring
- Viral load: at baseline and with modification of antiretroviral (ARV) treatment, at 2 to 8 weeks; then every 4 to 8 weeks until values are below the limit of detection (less than 200 copies/mL), then every 3 to 4 months; monitoring may be extended to every 6 months for patients who are immunologically stable, adherent, and with suppressed viral loads for more than 2 years.
- CD4 cell counts: at baseline and with modification of ARV treatment, then every 3 to 6 months during at least the first 2 years of treatment and if the CD4 counts are less than 300 cell/mm(3) or viremia develops; after 2 years monitoring may be extended to every 12 months for patients who are clinically stable with suppressed viral loads.
- Hepatitis B screening: baseline and with modification of ARV treatment; may repeat screening every 12 months if hepatitis B surface antigen or antibody are negative at baseline.
- Perform hepatitis C antibody testing prior to initiation or modification of ARV treatment; may repeat screening every 12 months in high-risk patients if results are negative at baseline.
- Improvement in symptoms and quality of life in patients with HIV infection may indicate efficacy.
- ALT, AST, and total bilirubin; at baseline and with modification of ARV treatment, at 2 to 8 weeks, then every 3 to 6 months; continue monitoring hepatic function closely for several months after discontinuation in patients co-infected with hepatitis B.
- Basic chemistry including serum sodium, potassium, bicarbonate, chloride, BUN, creatinine, and creatinine-based estimated glomerular filtration rate; at baseline and with modification of ARV treatment, at 2 to 8 weeks, then every 3 to 6 months.
- CBC with a differential; at baseline and with modification of ARV treatment, 6 months thereafter or every 3 to 6 months if CD4 testing is done.
- Fasting blood glucose or HbA1c at baseline and with modification of ARV treatment, then every 3 to 6 months in patients with abnormal values or every 12 months in patients with normal values.
- Fasting lipid profile at baseline and with modification of ARV treatment, then every 6 months in patients with abnormal values or annually in patients with normal values.
- Urinalysis: at baseline or modification of therapy, then annually.
- Pregnancy test, in women of reproductive potential; prior to therapy initiation.
- Estimate CrCl; at baseline and during treatment for all patients.
- Serum phosphorus, in patients with underlying chronic kidney disease.
- Signs and symptoms of hepatic dysfunction; for several months following therapy discontinuation for patients con-infected with HIV-1 and hepatitis B virus.
IV Compatibility
There is limited information regarding the compatibility of Bictegravir / emtricitabine / tenofovir alafenamide and IV administrations.
Overdosage
- No data are available on overdose of bictegravir /emtricitabine /tenofovir alafenamide in patients. If overdose occurs, monitor the patient for evidence of toxicity. Treatment of overdose with bictegravir /emtricitabine /tenofovir alafenamide consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient.
- Hemodialysis treatment removes approximately 30% of the FTC dose over a 3-hour dialysis period starting within 1.5 hours of FTC dosing (blood flow rate of 400 mL per minute and a dialysate flow rate of 600 mL per minute). It is not known whether FTC can be removed by peritoneal dialysis.
- Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%.
Pharmacology
Bictegravir/emtricitabine/tenofovir alafenamide
| |
| Combination of | |
| Bictegravir | integrase inhibitor |
| Emtricitabine | nucleoside reverse transcriptase inhibitor |
| Tenofovir alafenamide | nucleotide reverse transcriptase inhibitor |
| Identifiers | |
| CAS number | ? |
| ATC code | J05 |
| PubChem | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Bictegravir /emtricitabine /tenofovir alafenamide is a fixed dose combination of antiretroviral drugs bictegravir (BIC), emtricitabine (FTC), and tenofovir alafenamide (TAF).
Structure
Bictegravir

Emtricitabine

Tenofovir Alafenamide
Pharmacodynamics
Cardiac Electrophysiology
- In a thorough QT/QTc trial in 48 healthy subjects, BIC at doses 1.5 and 6 times the recommended dose did not affect the QT/QTc interval and did not prolong the PR interval. In a thorough QT/QTc trial in 48 healthy subjects, TAF at the recommended dose or at a dose 5 times the recommended dose, did not affect the QT/QTc interval and did not prolong the PR interval. The effect of FTC on the QT interval is not known.
Effects on Serum Creatinine
- Mean change from baseline in serum creatinine in healthy subjects who received BIC 75 mg (1.5 times the approved recommended dosage) once daily with food for 14 days was 0.1 mg per dL on Days 7 and 14 compared to placebo. BIC did not have a significant effect on the estimated creatinine clearance or on the actual glomerular filtration rate (determined by the clearance of probe drug, iohexol).
Pharmacokinetics
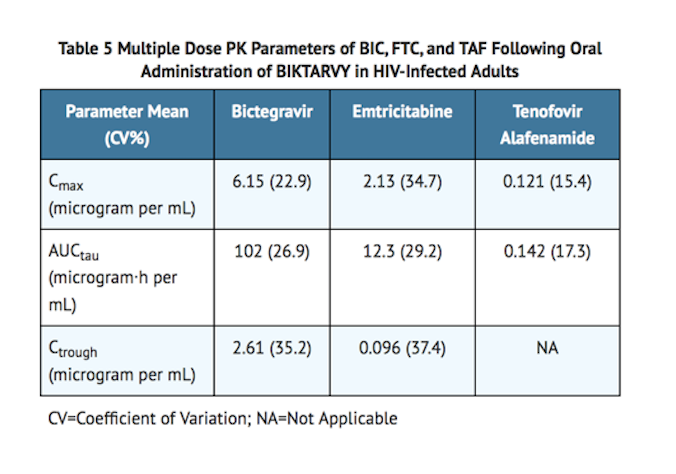
- The pharmacokinetic (PK) properties of bictegravir /emtricitabine /tenofovir alafenamide components are provided in Table 4. The multiple dose PK parameters of bictegravir /emtricitabine /tenofovir alafenamide components (based on population pharmacokinetic analysis) are provided in Table 5.

Specific Populations
Patients with Renal Impairment
- No clinically relevant differences in the pharmacokinetics of BIC, TAF, or its metabolite tenofovir were observed between subjects with severe renal impairment (CLcr 15 to 29 mL per minute estimated by Cockcroft-Gault method) and healthy subjects.
Patients with Hepatic Impairment
- Bictegravir: Clinically relevant changes in the pharmacokinetics of BIC were not observed in subjects with moderate (Child-Pugh Class B) hepatic impairment.
- Emtricitabine: The pharmacokinetics of FTC has not been studied in subjects with hepatic impairment; however, FTC is not significantly metabolized by liver enzymes, so the impact of hepatic impairment should be limited.
- Tenofovir Alafenamide: Clinically relevant changes in the pharmacokinetics of TAF or its metabolite tenofovir were not observed in subjects with mild or moderate (Child-Pugh Class A and B) hepatic impairment.
Hepatitis B and/or Hepatitis C Virus Coinfection
- The pharmacokinetics of BIC, FTC, and TAF have not been evaluated in subjects coinfected with hepatitis B and/or C virus.
Geriatric Patients
- The pharmacokinetics of BIC, FTC, and TAF have not been fully evaluated in the elderly (65 years of age and older). Population pharmacokinetics analysis of HIV-infected subjects in Phase 3 trials of bictegravir /emtricitabine /tenofovir alafenamide showed that age did not have a clinically relevant effect on exposures of BIC and TAF up to 74 years of age.
Race and Gender
- No clinically relevant changes in the pharmacokinetics of BIC, FTC, and TAF were observed based on gender or race.
Drug Interaction Studies
- As bictegravir /emtricitabine /tenofovir alafenamide is a complete regimen for the treatment of HIV-1 infection, comprehensive information regarding potential drug-drug interactions with other antiretroviral agents is not provided.
- BIC is a substrate of CYP3A and UGT1A1.
- BIC is an inhibitor of OCT2 and MATE1. At clinically relevant concentrations, BIC is not an inhibitor of hepatic transporters OATP1B1, OATP1B3, OCT1, BSEP, renal transporters OAT1 and OAT3, or CYP (including CYP3A) or UGT1A1 enzymes.
- TAF is a substrate of P-gp and BCRP.
- At clinically relevant concentrations, TAF is not an inhibitor of drug transporters P-gp, BCRP, hepatic transporters OATP1B1, OATP1B3, OCT1, BSEP, renal transporters OAT1, OAT3, OCT2, MATE1, or CYP (including CYP3A) or UGT1A1 enzymes.
- Drug interaction studies were conducted with bictegravir /emtricitabine /tenofovir alafenamide or its components. Tables 6 and 7 summarize the pharmacokinetic effects of other drugs on BIC and TAF, respectively. Table 8 summarizes the pharmacokinetic effects of bictegravir /emtricitabine /tenofovir alafenamide or its components on other drugs.
Effect of Other Drugs on Bictegravir /Emtricitabine /Tenofovir Alafenamide Components


Effect of Bictegravir /Emtricitabine /Tenofovir Alafenamide Components on Other Drugs

Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Bictegravir
- BIC was not carcinogenic in a 6-month rasH2 transgenic mouse study at doses of up to 100 mg/kg/day in males and 300 mg/kg/day in females. A carcinogenicity study in rats is ongoing.
- BIC was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
- BIC did not affect fertility, reproductive performance or embryonic viability in male and female rats at 29 times higher exposures (AUC) than in humans at the recommended dose of 50 mg BIC in bictegravir /emtricitabine /tenofovir alafenamide.
Emtricitabine
- In long-term carcinogenicity studies of FTC, no drug-related increases in tumor incidence were found in mice at doses up to 750 mg per kg per day (25 times the human systemic exposure at the recommended dose of 200 mg per day in bictegravir /emtricitabine /tenofovir alafenamide) or in rats at doses up to 600 mg per kg per day (30 times the human systemic exposure at the recommended dose in bictegravir /emtricitabine /tenofovir alafenamide).
- FTC was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or mouse micronucleus assays.
- FTC did not affect fertility in male rats at approximately 140 times or in male and female mice at approximately 60 times higher exposures (AUC) than in humans given the recommended 200 mg daily dose in bictegravir /emtricitabine /tenofovir alafenamide. Fertility was normal in the offspring of mice exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 60 times higher than human exposures at the recommended 200 mg daily dose in bictegravir /emtricitabine /tenofovir alafenamide.
Tenofovir Alafenamide
- Since TAF is rapidly converted to tenofovir and a lower tenofovir exposure in rats and mice was observed after TAF administration compared to TDF administration, carcinogenicity studies were conducted only with TDF. Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 10 times (mice) and 4 times (rats) those observed in humans at the recommended dose of TDF (300 mg) for HIV-1 infection. The tenofovir exposure in these studies was approximately 151 times (mice) and 51 times (rat) those observed in humans after administration of the daily recommended dose of bictegravir /emtricitabine /tenofovir alafenamide. At the high dose in female mice, liver adenomas were increased at tenofovir exposures approximately 10 times (300 mg TDF) and 151 times (bictegravir /emtricitabine /tenofovir alafenamide) the exposure observed in humans. In rats, the study was negative for carcinogenic findings.
- TAF was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
- There were no effects on fertility, mating performance or early embryonic development when TAF was administered to male rats at a dose equivalent to 155 times (25 mg TAF) the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 14 days prior to mating through Day 7 of gestation.
Animal Toxicology and/or Pharmacology
- Minimal to slight infiltration of mononuclear cells in the posterior uvea was observed in dogs with similar severity after three and nine month administration of TAF; reversibility was seen after a three month recovery period. No eye toxicity was observed in the dog at systemic exposures of 7 (TAF) and 14 (tenofovir) times the exposure seen in humans with the recommended daily TAF dose in bictegravir /emtricitabine /tenofovir alafenamide.
Clinical Studies
Description of Clinical Trials
- The efficacy and safety of bictegravir /emtricitabine /tenofovir alafenamide were evaluated in the trials summarized in Table 9.

Clinical Trial Results in HIV-1 Subjects with No Antiretroviral Treatment History
- In Trial 1489, subjects were randomized in a 1:1 ratio to receive either bictegravir /emtricitabine /tenofovir alafenamide (N=314) or ABC/DTG/3TC (600 mg/50 mg/300 mg) (N=315) once daily. In Trial 1490, subjects were randomized in a 1:1 ratio to receive either bictegravir /emtricitabine /tenofovir alafenamide (N=320) or DTG + FTC/TAF (50 mg + 200 mg/25 mg) (N=325) once daily.
- In Trial 1489, the mean age was 34 years (range 18–71), 90% were male, 57% were White, 36% were Black, and 3% were Asian. 22% percent of patients identified as Hispanic/Latino. The mean baseline plasma HIV-1 RNA was 4.4 log10 copies/mL (range 1.3–6.5). The mean baseline CD4+ cell count was 464 cells per mm3 (range 0–1424) and 11% had CD4+ cell counts less than 200 cells per mm3. 16% of subjects had baseline viral loads greater than 100,000 copies per mL.
- In Trial 1490, the mean age was 37 years (range 18–77), 88% were male, 59% were White, 31% were Black, and 3% were Asian. 25% percent of patients identified as Hispanic/Latino. The mean baseline plasma HIV-1 RNA was 4.4 log10 copies/mL (range 2.3–6.6). The mean baseline CD4+ cell count was 456 cells per mm3 (range 2–1636) and 12% had CD4+ cell counts less than 200 cells per mm3. 19% of subjects had baseline viral loads greater than 100,000 copies per mL.
- In both trials, subjects were stratified by baseline HIV-1 RNA (less than or equal to 100,000 copies per mL, greater than 100,000 copies per mL to less than or equal to 400,000 copies per mL, or greater than 400,000 copies per mL), by CD4 count (less than 50 cells per mm3, 50-199 cells per mm3, or greater than or equal to 200 cells per mm3), and by region (US or ex-US).
- Treatment outcomes of Trials 1489 and 1490 through Week 48 are presented in Table 10.

- Treatment outcomes were similar across subgroups by age, sex, race, baseline viral load, and baseline CD4+ cell count.
- In Trials 1489 and 1490, the mean increase from baseline in CD4+ count at Week 48 was 233 and 229 cells per mm3 in the bictegravir /emtricitabine /tenofovir alafenamide and ABC/DTG/3TC groups, respectively, and 180 and 201 cells per mm3 in the bictegravir /emtricitabine /tenofovir alafenamide and DTG + FTC/TAF groups, respectively.
Clinical Trial Results in HIV-1 Virologically-Suppressed Subjects Who Switched to Bictegravir /Emtricitabine /Tenofovir Alafenamide
- In Trial 1844, the efficacy and safety of switching from a regimen of DTG + ABC/3TC or ABC/DTG/3TC to bictegravir /emtricitabine /tenofovir alafenamide were evaluated in a randomized, double-blind trial of virologically-suppressed (HIV-1 RNA less than 50 copies per mL) HIV-1 infected adults (N=563, randomized and dosed). Subjects must have been stably suppressed (HIV-1 RNA less than 50 copies per mL) on their baseline regimen for at least 3 months prior to trial entry and had no history of treatment failure. Subjects were randomized in a 1:1 ratio to either switch to bictegravir /emtricitabine /tenofovir alafenamide at baseline (N=282), or stay on their baseline antiretroviral regimen (N=281). Subjects had a mean age of 45 years (range 20–71), 89% were male, 73% were White, and 22% were Black. 17% of subjects identified as Hispanic/Latino. The mean baseline CD4+ cell count was 723 cells per mm3 (range 124–2444).
- In Trial 1878, the efficacy and safety of switching from either ABC/3TC or FTC/TDF (200/300 mg) plus ATV or DRV (given with either cobicistat or ritonavir) to bictegravir /emtricitabine /tenofovir alafenamide were evaluated in a randomized, open-label study of virologically-suppressed HIV-1 infected adults (N=577, randomized and dosed). Subjects must have been stably suppressed on their baseline regimen for at least 6 months, must not have been previously treated with any INSTI, and had no history of treatment failure. Subjects were randomized in a 1:1 ratio to either switch to bictegravir /emtricitabine /tenofovir alafenamide (N=290) or stay on their baseline antiretroviral regimen (N=287). Subjects had a mean age of 46 years (range 20–79), 83% were male, 66% were White, and 26% were Black. 19% of subjects identified as Hispanic/Latino. The mean baseline CD4+ cell count was 663 cells per mm3 (range 62–2582). Subjects were stratified by prior treatment regimen. At screening, 15% of subjects were receiving ABC/3TC plus ATV or DRV (given with either cobicistat or ritonavir) and 85% of subjects were receiving FTC/TDF plus ATV or DRV (given with either cobicistat or ritonavir).
- Treatment outcomes of Trials 1844 and 1878 through Week 48 are presented in Table 11.

- In Trial 1844, treatment outcomes between treatment groups were similar across subgroups by age, sex, race, and region. The mean change from baseline in CD4+ count at Week 48 was -31 cells per mm3 in subjects who switched to bictegravir /emtricitabine /tenofovir alafenamide and 4 cells per mm3 in subjects who stayed on ABC/DTG/3TC.
- In Trial 1878, treatment outcomes between treatment groups were similar across subgroups by age, sex, race, and region. The mean change from baseline in CD4+ count at Week 48 was 25 cells per mm3 in patients who switched to bictegravir /emtricitabine /tenofovir alafenamide and 0 cells per mm3 in patients who stayed on their baseline regimen.
How Supplied
- Bictegravir /Emtricitabine /Tenofovir Alafenamide tablets are purplish brown, capsule-shaped, and film-coated with "GSI" debossed on one side and "9883" on the other side. Each bottle contains 30 tablets (NDC 61958-2501-1), a silica gel desiccant, polyester coil, and is closed with a child-resistant closure. Each bictegravir /emtricitabine /tenofovir alafenamide tablet contains 50 mg of bictegravir (BIC), 200 mg of emtricitabine (FTC), and 25 mg of tenofovir alafenamide (TAF).
Storage
- Store below 30 °C (86 °F).
- Keep container tightly closed.
- Dispense only in original container.
Images
Drug Images
{{#ask: Page Name::Bictegravir / emtricitabine / tenofovir alafenamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Bictegravir / emtricitabine / tenofovir alafenamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Post-treatment Acute Exacerbation of Hepatitis B in Patients with HBV Coinfection
- Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued products containing FTC and/or TDF, and may likewise occur with discontinuation of bictegravir /emtricitabine /tenofovir alafenamide. Advise the patient to not discontinue bictegravir /emtricitabine /tenofovir alafenamide without first informing their healthcare provider.
Drug Interactions
- Bictegravir /emtricitabine /tenofovir alafenamide may interact with certain drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or non-prescription medication or herbal products including St. John's wort.
Immune Reconstitution Syndrome
- Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started.
Renal Impairment
- Advise patients to avoid taking bictegravir /emtricitabine /tenofovir alafenamide with concurrent or recent use of nephrotoxic agents. Renal impairment including cases of acute renal failure has been reported in association with the use of tenofovir prodrugs.
Lactic Acidosis and Severe Hepatomegaly
- Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with use of drugs similar to bictegravir /emtricitabine /tenofovir alafenamide. Advise patients that they should stop bictegravir /emtricitabine /tenofovir alafenamide if they develop clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity.
Missed Dosage
- Inform patients that it is important to take bictegravir /emtricitabine /tenofovir alafenamide on a regular dosing schedule with or without food and to avoid missing doses as it can result in development of resistance.
Pregnancy Registry
- Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to bictegravir /emtricitabine /tenofovir alafenamide.
Lactation
- Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk.

Precautions with Alcohol
Alcohol-Bictegravir / emtricitabine / tenofovir alafenamide interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Biktarvy
Look-Alike Drug Names
There is limited information regarding Bictegravir / emtricitabine / tenofovir alafenamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.