Aryne
In chemistry, an aryne is an uncharged reactive intermediate derived from an aromatic system by removal of two ortho substituents, leaving two orbitals with two electrons distributed between them [1]. In analogy with carbenes and nitrenes, an aryne has a singlet state and a triplet state. The name benzyne (1) C6H4 for the simplest aryne is open for criticism because it implies an alkyne bond which is not the case, a better name is dehydrobenzene. Benzyne is stabilized by resonance between structures 1 and 2—a better representation of the electronic structure is 3. The "extra" pi bond (4b) is localised and orthogonal to the other pi bonds making up the atomatic ring (4a). Benzyne can also be drawn as a diradical: the pi bond 4b splits homolytically, leaving one electron on each of the two atoms that are formally part of that bond.
Benzyne is an extremely reactive species due to the nature of its triple bond. In normal acetylenic species (such as the simplest, ethyne) the unhybridized p orbitals are parallel to one another above and below the molecular axis. This facilitates maximum orbital overlap. In benzyne, however, the p orbtials are distored to accommodate the triple bond within the ring system, reducing their effective overlap. A suitable chemical trap for benzyne is a cyclopentadiene.
Benzyne can be a ortho-, meta- and para-benzyne where the biradical can be a 1,2-, 1,3- and 1,4-biradical species respectively. Their energies in silico are respectively 106, 122, and 138 kcal/mole .[2]. The 1,4-biradical species has been identified in the Bergman cyclization. Professor Maitland Jones of Princeton University has studied the interconversion of the ortho-, meta - and para-benzynes.[2][3]
Aryne chemistry
Arynes are prepared from electron deficient aromatic compounds (often aryl halides) in presence of a strong base. The most prominent aryne reactions are Diels-Alder reactions with dienes. Tetrabromobenzene reacts with butyllithium to the diaryne intermediate with furan to form a tetrahydroanthracene [4]. The mixture of syn and anti conformers can be separated based on difference in methanol solubility.
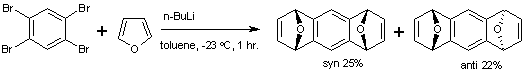
Anthracene is converted to a triptycene by Diels-Alder reaction of an aryne with the central benzene ring [5]. A pentiptycene is the anthracene analogue after reaction with the diaryne.
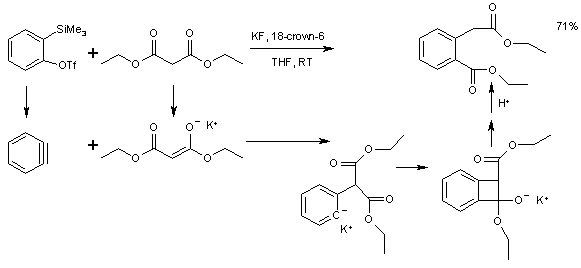
Aryne reactivity can also be extended to carbon to carbon insertion reactions into substrates that can react both as a nucleophile and as an electrophile with for instance a malonic acid ester [6]. The precursor to benzyne in this reaction is 2-(Trimethylsilyl)phenyl triflate.
Aryne interconversions
An ortho to meta-aryne conversion has been postulated to occur in the pyrolysis (900°C) of the phenyl substituted aryne precursor 1 [2] to acenaphthylene 7.
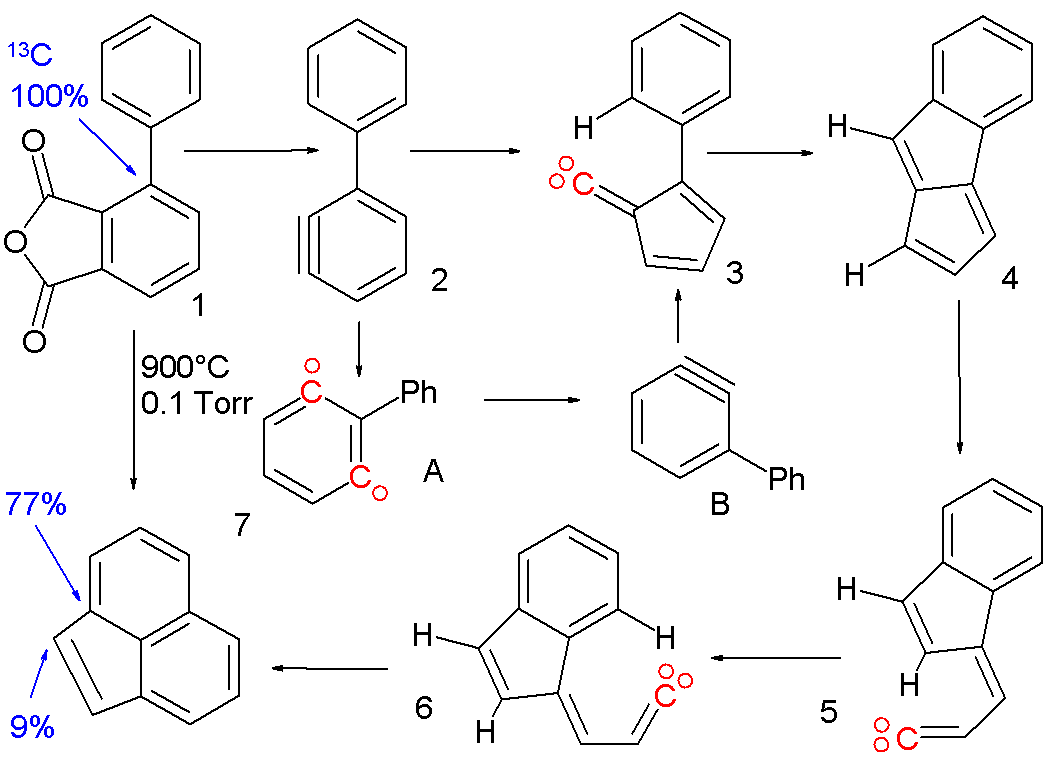
This reaction takes place through several reactive intermediates: the aryne 2 is formed from phenyl substituted phthalic anhydride which rearranges with ring contraction to the vinylidene 3. This carbene gives a C-H insertion reaction to pentalene 4 and then a retro insertion to vinylidene 5. After a cis-trans isomerism to 6 a final insertion reaction gives the acenaphthylene. Evidence for a phenyl migration in aryne 2 from the o-aryne to the m-aryne is based on isotope scrambling. When the ipso carbon atom is replaced by 13C in the precursor molecule it will in the default mechanism again show up in the acenaphthylene in an ipso arene position. The presence of 13C in the bridge position can only be explained when 15% of 2 isomerizes to m-aryne A.
Scope
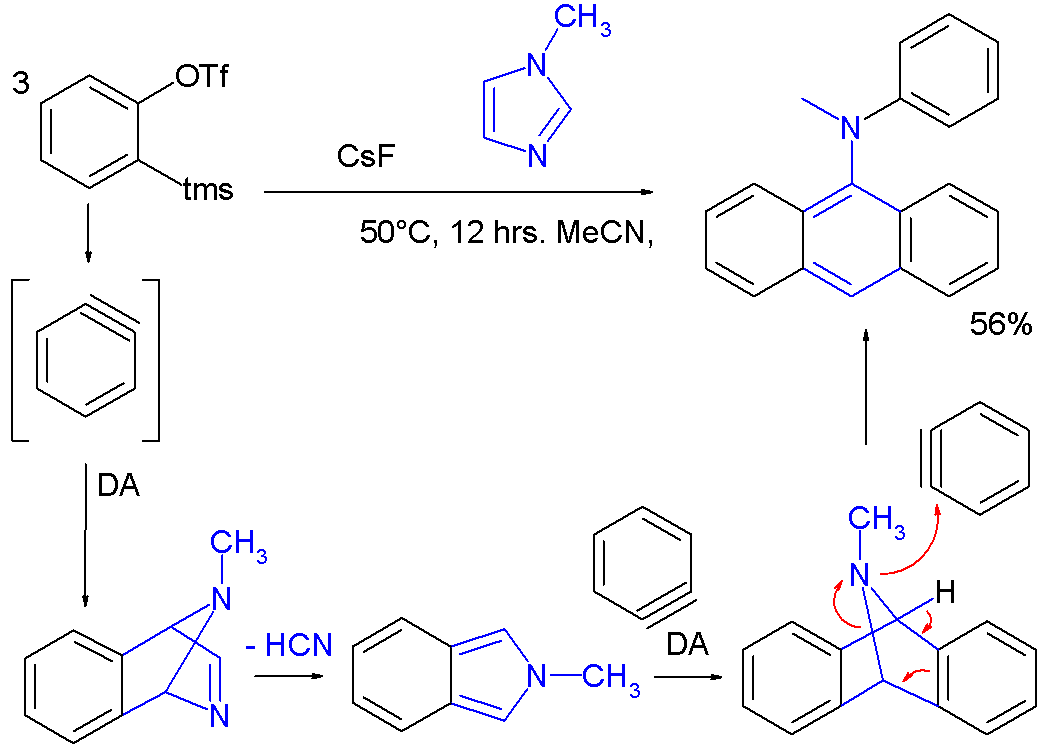
Aryne chemistry has been applied to the synthesis of novel aryl amines in a tandem reaction including two Diels-Alder reactions with three benzyne molecules reacting to one imidazole molecule [7]:
See also
- More examples use of aryne chemistry: tricyclobutabenzene
References
- ↑ Gilchrist T.C.; Rees C.W.; (1969) Carbenes, Nitrenes and Arynes. Nelson. London.
- ↑ 2.0 2.1 2.2 A m-Benzyne to o-Benzyne Conversion Through a 1,2-Shift of a Phenyl Group. Blake, M. E.; Bartlett, K. L.; Jones, M. Jr. J. Am. Chem. Soc. 2003, 125, 6485. doi:10.1021/ja0213672
- ↑ A p-Benzyne to m-Benzyne Conversion Through a 1,2-Shift of a Phenyl Group. Completion of the Benzyne Cascade, Polishchuk, A. L.; Bartlett, K. L.; Friedman, L. A.; Jones, M. Jr. J. Phys. Org. Chem. 2004, Volume 17, Issue 9 , Pages 798 - 806. doi:10.1002/poc.797
- ↑ Organic Syntheses, Coll. Vol. 10, p.678; Vol. 75, p.201 Article
- ↑ Iptycenes, cuppedophanes and cappedophanes Harold Hart Pure & App Chem, Vol. 65, No. 1, pp. 27-34, 1993. Article
- ↑ Facile insertion reaction of arynes into carbon–carbon -bonds Hiroto Yoshida, Masahiko Watanabe, Joji Ohshita and Atsutaka Kunai, Chem. Commun., 2005, (26), 3292 Abstract
- ↑ A New Tandem Reaction of Benzyne: One-Pot Synthesis of Aryl Amines Containing Anthracene Chunsong Xie and Yuhong Zhang Org. Lett.; 2007; 9(5) pp 781 - 784; (Letter) doi:10.1021/ol063017g