Fremanezumab-vfrm
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Fremanezumab-vfrm is a calcitonin gene-related peptide antagonist that is FDA approved for the preventive treatment of migraine in adults. Common adverse reactions include injection site reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Fremanezumab-vfrm is indicated for the preventive treatment of migraine in adults.
Dosage
- Two subcutaneous dosing options of fremanezumab-vfrm are available to administer the recommended dosage:
- 225 mg monthly, or
- 675 mg every 3 months (quarterly), which is administered as three consecutive subcutaneous injections of 225 mg each.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding fremanezumab-vfrm Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding fremanezumab-vfrm Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding fremanezumab-vfrm Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding fremanezumab-vfrm Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- Fremanezumab-vfrm is contraindicated in patients with serious hypersensitivity to fremanezumab-vfrm or to any of the excipients.
Warnings
Hypersensitivity Reactions
- Hypersensitivity reactions, including rash, pruritus, drug hypersensitivity, and urticaria, were reported with fremanezumab-vfrm in clinical trials. Most reactions were mild to moderate, but some led to discontinuation or required corticosteroid treatment. Most reactions were reported from within hours to one month after administration.
- If a hypersensitivity reaction occurs, consider discontinuing fremanezumab-vfrm, and institute appropriate therapy.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in clinical practice.
- The safety of fremanezumab-vfrm was evaluated in 2512 patients with migraine who received at least 1 dose of fremanezumab-vfrm, representing 1279 patient-years of exposure. Of these, 1730 patients were exposed to fremanezumab-vfrm 225 mg monthly or fremanezumab-vfrm 675 mg quarterly for at least 6 months, 775 patients for at least 12 months, and 138 patients for at least 15 months. In placebo-controlled clinical trials (Studies 1 and 2), 662 patients received fremanezumab-vfrm 225 mg monthly for 12 weeks (with or without a loading dose of 675 mg), and 663 patients received fremanezumab-vfrm 675 mg quarterly for 12 weeks. In the controlled trials, 87% of patients were female, 80% were White, and the mean age was 41 years.
- The most common adverse reactions in the clinical trials for the preventive treatment of migraine (incidence at least 5% and greater than placebo) were injection site reactions. The adverse reactions that most commonly led to discontinuations were injection site reactions (1%). Table 1 summarizes adverse reactions reported in the 3-month placebo-controlled studies (Study 1 and Study 2), and the 1-month follow-up period after those studies.

Immunogenicity
- As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to fremanezumab-vfrm in the studies described below with the incidence of antibodies in other studies to other products may be misleading. Clinical immunogenicity of fremanezumab-vfrm was monitored by analyzing anti-drug antibodies (ADA) and neutralizing antibodies in drug-treated patients. The data reflect the percentage of patients whose test results were positive for antibodies to fremanezumab-vfrm in specific assays.
- In 3-month placebo-controlled studies, treatment-emergent ADA responses were observed in 6 out of 1701 (0.4%) fremanezumab-vfrm-treated patients. One of the 6 patients developed anti-fremanezumab-vfrm neutralizing antibodies at Day 84. In the ongoing long-term open-label study, ADA were detected in 1.6% of patients (30 out of 1888). Out of 30 ADA-positive patients, 17 had a neutralizing activity in their post-dose samples. Although these data do not demonstrate an impact of anti-fremanezumab-vfrm antibody development on the efficacy or safety of fremanezumab-vfrm in these patients, the available data are too limited to make definitive conclusions.
Postmarketing Experience
There is limited information regarding Fremanezumab-vfrm Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Fremanezumab-vfrm Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no adequate data on the developmental risk associated with the use of fremanezumab-vfrm in pregnant women. Fremanezumab-vfrm has a long half-life. This should be taken into consideration for women who are pregnant or plan to become pregnant while using fremanezumab-vfrm. Administration of fremanezumab-vfrm to rats and rabbits during the period of organogenesis or to rats throughout pregnancy and lactation at doses resulting in plasma levels greater than those expected clinically did not result in adverse effects on development [see Animal Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The estimated rate of major birth defects (2.2-2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
- Published data have suggested that women with migraine may be at increased risk of preeclampsia during pregnancy.
Animal Data
- When fremanezumab-vfrm (0, 50, 100, or 200 mg/kg) was administered to male and female rats by weekly subcutaneous injection prior to and during mating and continuing in females throughout organogenesis, no adverse embryofetal effects were observed. The highest dose tested was associated with plasma exposures (AUC) approximately 2 times that in humans at a dose of 675 mg.
- Administration of fremanezumab-vfrm (0, 10, 50, or 100 mg/kg) weekly by subcutaneous injection to pregnant rabbits throughout the period of organogenesis produced no adverse effects on embryofetal development. The highest dose tested was associated with plasma AUC approximately 3 times that in humans (675 mg).
- Administration of fremanezumab-vfrm (0, 50, 100, or 200 mg/kg) weekly by subcutaneous injection to female rats throughout pregnancy and lactation resulted in no adverse effects on pre- and postnatal development. The highest dose tested was associated with plasma AUC approximately 2 times that in humans (675 mg).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Fremanezumab-vfrm in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Fremanezumab-vfrm during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of fremanezumab-vfrm in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for fremanezumab-vfrm and any potential adverse effects on the breastfed infant from fremanezumab-vfrm or from the underlying maternal condition.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of fremanezumab-vfrm did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Fremanezumab-vfrm with respect to specific gender populations.
Race
There is no FDA guidance on the use of Fremanezumab-vfrm with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Fremanezumab-vfrm in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Fremanezumab-vfrm in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Fremanezumab-vfrm in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Fremanezumab-vfrm in patients who are immunocompromised.
Administration and Monitoring
Administration
Recommended Dosage
- Two subcutaneous dosing options of fremanezumab-vfrm are available to administer the recommended dosage:
- 225 mg monthly, or
- 675 mg every 3 months (quarterly), which is administered as three consecutive subcutaneous injections of 225 mg each.
- When switching dosage options, administer the first dose of the new regimen on the next scheduled date of administration. If a dose of fremanezumab-vfrm is missed, administer as soon as possible. Thereafter, fremanezumab-vfrm can be scheduled from the date of the last dose.
Important Administration Instructions
- Fremanezumab-vfrm is for subcutaneous use only.
- Fremanezumab-vfrm may be administered by healthcare professionals, patients, and/or caregivers. Prior to use, provide proper training to patients and/or caregivers on the preparation and administration of fremanezumab-vfrm prefilled syringe, including aseptic technique:
- Remove fremanezumab-vfrm from the refrigerator. Prior to use, allow fremanezumab-vfrm to sit at room temperature for 30 minutes protected from direct sunlight. Do not warm by using a heat source such as hot water or a microwave. Do not use fremanezumab-vfrm if it has been at room temperature for 24 hours or longer.
- Follow aseptic injection technique every time fremanezumab-vfrm is administered.
- Inspect fremanezumab-vfrm for particles or discoloration prior to administration. Do not use if the solution is cloudy, discolored, or contains particles.
- Administer fremanezumab-vfrm by subcutaneous injection into areas of the abdomen, thigh, or upper arm that are not tender, bruised, red, or indurated. For multiple injections, you may use the same body site, but not the exact location of the previous injection.
- Do not co-administer fremanezumab-vfrm with other injectable drugs at the same injection site.
Monitoring
There is limited information regarding Fremanezumab-vfrm Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Fremanezumab-vfrm and IV administrations.
Overdosage
There is limited information regarding Fremanezumab-vfrm overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Fremanezumab-vfrm?
| |
| Therapeutic monoclonal antibody | |
| Source | zu |
| Target | CGRP α, β |
| Identifiers | |
| CAS number | |
| ATC code | N02 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | TEV-48125 |
| Pharmacokinetic data | |
| Bioavailability | 55–66% |
| Metabolism | Proteolysis |
| Half life | 30–31 days (estimated) |
| Excretion | Kidney |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Subcutaneous injection |
Mechanism of Action
- Fremanezumab-vfrm is a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor.
Structure
- Fremanezumab-vfrm is a fully humanized IgG2Δa/kappa monoclonal antibody specific for calcitonin gene-related peptide (CGRP) ligand. Fremanezumab-vfrm is produced by recombinant DNA technology in Chinese hamster ovary (CHO) cells. The antibody consists of 1324 amino acids and has a molecular weight of approximately 148 kDa.
Pharmacodynamics
- The relationship between the pharmacodynamic activity and the mechanism(s) by which fremanezumab-vfrm exerts its clinical effects is unknown.
Pharmacokinetics
Absorption
- After single subcutaneous (SC) administrations of 225 mg, 675 mg, and 900 mg fremanezumab-vfrm, median time to maximum concentrations (tmax) was 5 to 7 days. Dose-proportionality, based on population PK, was observed between 225 mg to 900 mg. Steady state was achieved by approximately 168 days (about 6 months) following 225 mg SC monthly and 675 mg SC quarterly dosing regimens. Median accumulation ratio, based on once-monthly and once-quarterly dosing regimens, is approximately 2.3 and 1.2, respectively.
Distribution
- Fremanezumab-vfrm has an apparent volume of distribution of approximately 6 liters, suggesting minimal distribution to the extravascular tissues.
Metabolism
- Similar to other monoclonal antibodies, fremanezumab-vfrm is degraded by enzymatic proteolysis into small peptides and amino acids.
Elimination
- Fremanezumab-vfrm apparent clearance was approximately 0.141 L/day. Fremanezumab-vfrm was estimated to have a half-life of approximately 31 days.
Specific Populations
- A population PK analysis assessing effects of age, race, sex, and weight was conducted on data from 2287 subjects. No dose adjustments are recommended for fremanezumab-vfrm.
Patients with Hepatic or Renal Impairment
- Hepatic/renal impairment is not expected to affect the pharmacokinetics of fremanezumab. A population PK analysis of integrated data from the fremanezumab-vfrm clinical studies did not reveal a difference in the pharmacokinetics of fremanezumab in patients with mild hepatic impairment, relative to those with normal hepatic function. There were only 4 patients with moderate hepatic impairment, and no patient with severe hepatic impairment in fremanezumab clinical studies. No dedicated hepatic/renal impairment studies were conducted to assess the effect of hepatic or renal impairment on the pharmacokinetics of fremanezumab.
Drug Interactions
- Fremanezumab is not metabolized by cytochrome P450 enzymes; therefore, interactions with concomitant medications that are substrates, inducers, or inhibitors of cytochrome P450 enzymes are unlikely. Additionally, the effects of medications for the acute treatment (specifically analgesics, ergots, and triptans) and preventive treatment of migraine were evaluated in a population PK model, and found not to influence fremanezumab exposure.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Carcinogenicity studies of fremanezumab-vfrm were not conducted.
Mutagenesis
- Genetic toxicology studies of fremanezumab-vfrm were not conducted.
Impairment of Fertility
- When fremanezumab-vfrm (0, 50, 100, or 200 mg/kg) was administered to male and female rats by weekly subcutaneous injection prior to and during mating and continuing in females throughout organogenesis, no adverse effects on male or female fertility were observed. The highest dose tested was associated with plasma exposures (AUC) approximately 2 times that in humans at a dose of 675 mg.
Clinical Studies
- The efficacy of fremanezumab-vfrm was evaluated as a preventive treatment of episodic or chronic migraine in two multicenter, randomized, 3-month, double-blind, placebo-controlled studies (Study 1 and Study 2, respectively).
Episodic Migraine
- Study 1 (NCT 02629861) included adults with a history of episodic migraine (patients with <15 headache days per month). All patients were randomized (1:1:1) to receive subcutaneous injections of either fremanezumab-vfrm 675 mg every three months (quarterly), fremanezumab-vfrm 225 mg monthly, or placebo monthly, over a 3-month treatment period. Patients were allowed to use acute headache treatments during the study. A subset of patients (21%) was allowed to use one additional concomitant preventive medication.
- The study excluded patients with a history of significant cardiovascular disease, vascular ischemia, or thrombotic events, such as cerebrovascular accident, transient ischemic attacks, deep vein thrombosis, or pulmonary embolism.
- The primary efficacy endpoint was the mean change from baseline in the monthly average number of migraine days during the 3-month treatment period. Secondary endpoints included the proportion of patients reaching at least a 50% reduction in monthly average number of migraine days during the 3-month treatment period, the mean change from baseline in the monthly average number of days of use of any acute headache medication during the 3-month treatment period, and the mean change from baseline in the number of migraine days during the first month of the treatment period.
- In Study 1, a total of 875 patients (742 females, 133 males), ranging in age from 18 to 70 years, were randomized. A total of 791 patients completed the 3-month double-blind phase. The mean migraine frequency at baseline was approximately 9 migraine days per month, and was similar across treatment groups.
- Both monthly and quarterly dosing regimens of fremanezumab-vfrm demonstrated statistically significant improvements for efficacy endpoints compared to placebo over the 3-month period, as summarized in Table 2.

- Figure 1 displays the mean change from baseline in the average monthly number of migraine days in Study 1.

- Figure 2 shows the distribution of change from baseline in mean monthly migraine days in bins of 2 days by treatment group in Study 1. A treatment benefit over placebo for both doses of fremanezumab-vfrm is seen across a range of changes from baseline in monthly migraine days.

- Study 2 (NCT 02621931) included adults with a history of chronic migraine (patients with ≥15 headache days per month). All patients were randomized (1:1:1) to receive subcutaneous injections of either fremanezumab-vfrm 675 mg starting dose followed by 225 mg monthly, 675 mg every 3 months (quarterly), or placebo monthly, over a 3-month treatment period. Patients were allowed to use acute headache treatments during the study. A subset of patients (21%) was allowed to use one additional concomitant, preventive medication.
- The study excluded patients with a history of significant cardiovascular disease, vascular ischemia, or thrombotic events, such as cerebrovascular accident, transient ischemic attacks, deep vein thrombosis, or pulmonary embolism.
- The primary efficacy endpoint was the mean change from baseline in the monthly average number of headache days of at least moderate severity during the 3-month treatment period. The secondary endpoints were the mean change from baseline in the monthly average number of migraine days during the 3-month treatment period, the proportion of patients reaching at least 50% reduction in the monthly average number of headache days of at least moderate severity during the 3-month treatment period, the mean change from baseline in the monthly average number of days of use of any acute headache medication during the 3-month treatment period, and the mean change from baseline in the number of headache days of at least moderate severity during the first month of treatment.
- In Study 2, a total of 1130 patients (991 females, 139 males), ranging in age from 18 to 70 years, were randomized. A total of 1034 patients completed the 3-month double-blind phase.
- Both monthly and quarterly dosing regimens of fremanezumab-vfrm treatment demonstrated statistically significant improvement for key efficacy outcomes compared to placebo, as summarized in Table 3.
- Figure 3 displays the mean change from baseline in the average monthly number of headache days of at least moderate severity in Study 2.

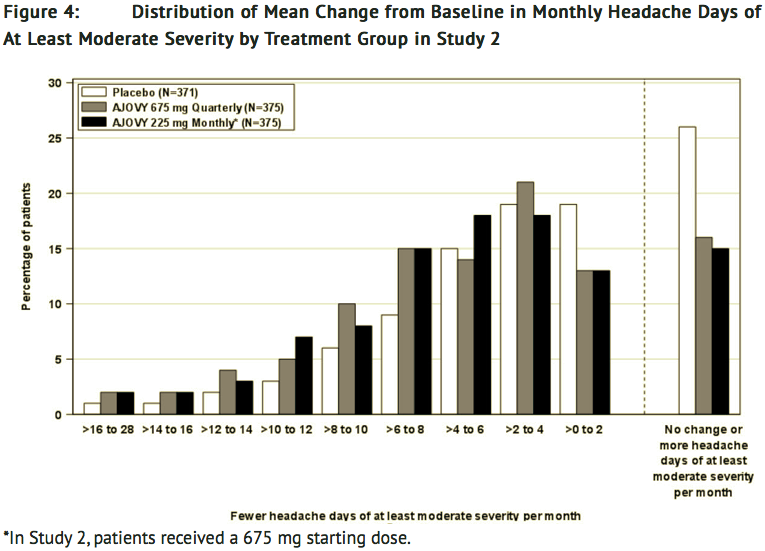
- Figure 4 shows the distribution of change from baseline in monthly headache days of at least moderate severity at month 3 in bins of 3 days by treatment group. A treatment benefit over placebo for both dosing regimens of fremanezumab-vfrm is seen across a range of changes from baseline in headache days.
How Supplied
- Fremanezumab-vfrm injection is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution for subcutaneous administration.
- The prefilled syringe cap is not made with natural rubber latex.
- Fremanezumab-vfrm is supplied as follows:
- NDC 51759-204-10: carton of one 225 mg/1.5 mL single-dose prefilled syringe
Storage
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original outer carton to protect from light.
- If necessary, fremanezumab-vfrm may be kept in the original carton at room temperature up to 25°C (77°F) for a maximum of 24 hours. After removal from the refrigerator, fremanezumab-vfrm must be used within 24 hours or discarded.
- Do not freeze.
- Do not expose to extreme heat or direct sunlight.
- Do not shake.
Images
Drug Images
{{#ask: Page Name::Fremanezumab-vfrm |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Fremanezumab-vfrm |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information on Preparation and Administration
- Provide guidance to patients and caregivers on proper subcutaneous administration technique, including aseptic technique, and how to use the single-dose prefilled syringe. Instruct patients and/or caregivers to read and follow the Instructions for Use each time they use fremanezumab-vfrm.
- Instruct patients prescribed the regimen of 675 mg every 3 months to administer the dosage as three consecutive subcutaneous injections of 225 mg each.
Hypersensitivity Reactions
- Inform patients about the signs and symptoms of hypersensitivity reactions and that these reactions can occur up to 1 month after administration. Advise patients to contact their healthcare provider immediately if signs or symptoms of hypersensitivity reactions occur.
Medication Guide

Instructions for Use

Precautions with Alcohol
Alcohol-Fremanezumab-vfrm interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Fremanezumab-vfrm Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.