Vilazodone
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Vilazodone is a antidepressant, serotonine agonist that is FDA approved for the treatment of major depressive disorder (MDD). Common adverse reactions include diarrhea, nausea, vomiting, xerostomia, dizziness, insomnia..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Vilazodone FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vilazodone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vilazodone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Vilazodone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vilazodone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vilazodone in pediatric patients.
Contraindications
Monoamine Oxidase Inhibitors (MAOIs)
The use of MAOIs intended to treat psychiatric disorders with VIIBRYD or within 14 days of stopping treatment with VIIBRYD is contraindicated because of an increased risk of serotonin syndrome. The use of VIIBRYD within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated.
Starting VIIBRYD in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome.
Warnings
There is limited information regarding Vilazodone Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
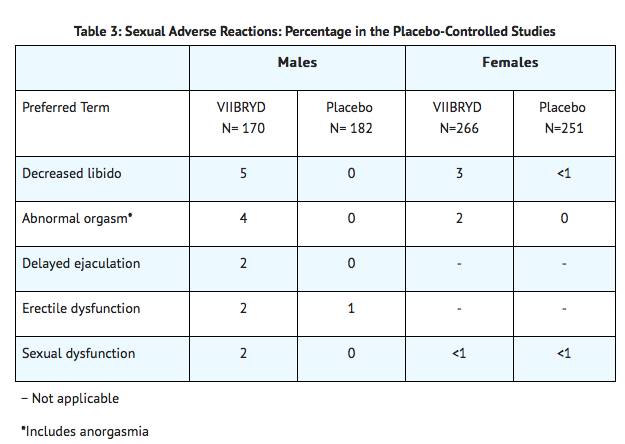
The most commonly observed adverse reactions in VIIBRYD-treated MDD patients in placebo-controlled studies (incidence ≥ 5% and at least twice the rate of placebo) were: diarrhea, nausea, vomiting, and insomnia.
Patient Exposure
The safety of VIIBRYD was evaluated in 2,177 patients (18-70 years of age) diagnosed with MDD who participated in clinical studies, representing 552 patient-years of exposure. In an open-label 52 week study at 40 mg daily, 599 patients were exposed to VIIBRYD for a total of 348 patient-years. The information presented in these sections was derived from studies of VIIBRYD 40 mg daily in major depressive disorder including: 1) 2 placebo-controlled 8-week studies in 861 patients, including 436 receiving vilazodone; and 2) an open-label 52-week study of 599 patients. These studies included a titration period of 10 mg daily for 7 days followed by 20 mg daily for 7 days. In these clinical trials, VIIBRYD was administered with food. Because clinical trials are conducted under widely varying conditions and varying lengths of time, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect rates observed in practice.
Adverse reactions reported as reasons for discontinuation of treatment
In the placebo-controlled studies of MDD there was no single adverse reaction leading to discontinuation in > 1% of the patients. Overall, 7.1% of the patients who received VIIBRYD discontinued treatment due to an adverse reaction, compared with 3.2% of placebo-treated patients in these studies.
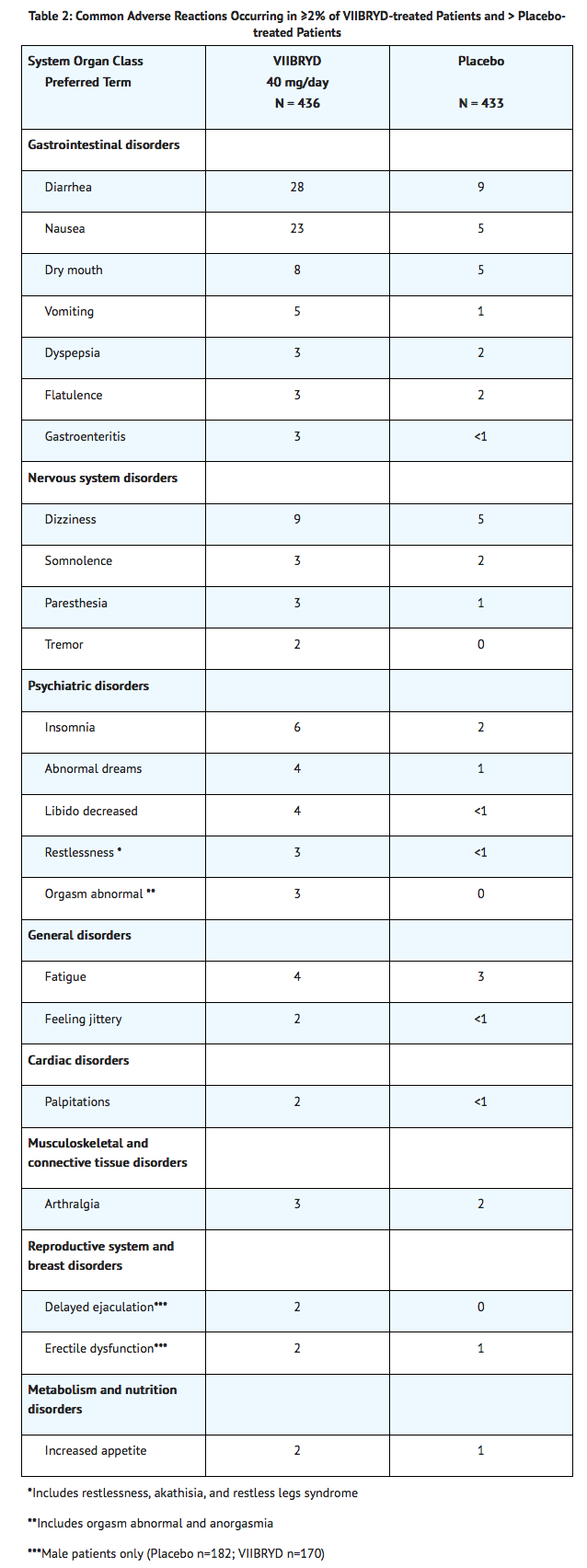
Common adverse reactions in placebo-controlled MDD studies
Table 2 shows the incidence of common adverse reactions that occurred in ≥ 2% of VIIBRYD-treated MDD patients (and greater than in placebo-treated patients) in the placebo-controlled studies.
Laboratory Tests
VIIBRYD has not been associated with any clinically important changes in laboratory test parameters in serum chemistry (including liver function tests), hematology and urinalysis, as measured in placebo-controlled studies. These studies include analysis of mean change from baseline and the proportion of patients meeting criteria for potentially clinically significant changes from baseline. Results from a 52-week open-label study were consistent with the findings from the placebo-controlled studies.
ECG
VIIBRYD has not been associated with any clinically significant effect on ECG parameters, including QT, QTc, PR and QRS intervals, or with any arrhythmogenic potential. ECGs were evaluated in a thorough QTc study at doses up to 80 mg daily with food and in the placebo-controlled studies.
Vital Signs
VIIBRYD has not been associated with any clinically significant effect on vital signs, including systolic and diastolic blood pressure and heart rate, as measured in placebo-controlled studies. These studies included analyses of (1) change from baseline, and (2) the proportion of patients meeting criteria for potentially clinically significant changes from baseline. Results from a 52-week open-label study were consistent with the findings from the placebo-controlled studies.
Weight
VIIBRYD had no effect on body weight as measured by the mean change from baseline in the 8-week, placebo-controlled studies. The mean changes in weight were +0.16 kg in the VIIBRYD group and +0.18 kg in the placebo group. The proportions of patients with a weight gain ≥ 7% were 0.9% in the VIIBRYD group and 1.2% in the placebo group. The proportions of patients with a weight decrease ≥ 7% were 1.4% in the VIIBRYD group and 1.4% in the placebo group.
Other adverse reactions observed in clinical studies
The following listing does not include reactions:
1) already listed in previous tables or elsewhere in labeling
2) for which a drug cause was remote
3) which were so general as to be uninformative
4) which were not considered to have significant clinical implications
5) which occurred at a rate equal to or less than placebo
Reactions are categorized by body system according to the following definitions: frequent adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare reactions are those occurring in fewer than 1/1000 patients:
Cardiac Disorders
- Infrequent: Ventricular extrasystoles
Eye Disorders
- Frequent: vision blurred, dry eye.
- Infrequent: cataracts.
General disorders
- Infrequent: feeling abnormal
Metabolism and Nutrition Disorders
- Frequent: decreased appetite
Nervous System
Psychiatric disorders
- Infrequent: Panic attack, mania
Renal and Urinary Disorder
Infrequent: pollakiuria
Skin and subcutaneous tissue disorders
- Frequent: hyperhidrosis, night sweats
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of VIIBRYD. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure. These events include:
General Disorders and administrative site conditions
Psychiatric Disorders
Drug Interactions
Central Nervous System (CNS)-Active Agents
The risk of using VIIBRYD in combination with other CNS-active drugs has not been systematically evaluated. Consequently, use caution when VIIBRYD is prescribed in combination with other CNS-active drugs.
Monoamine Oxidase Inhibitors (MAOIs)
Serotonergic Drugs
=====Drugs that Interfere with Hemostasis===== (e.g., NSAIDs, Aspirin, and Warfarin) Serotonin release by platelets plays an important role in hemostasis. Epidemiological studies of case-control and cohort design have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. These studies have also shown that concurrent use of an NSAID or aspirin may potentiate this risk of bleeding. Altered anticoagulant effects, including increased bleeding, have been reported when SSRIs and SNRIs are co-administered with warfarin. Patients receiving warfarin therapy should be carefully monitored when VIIBRYD is initiated or discontinued.
Potential for Other Drugs to Affect Vilazodone
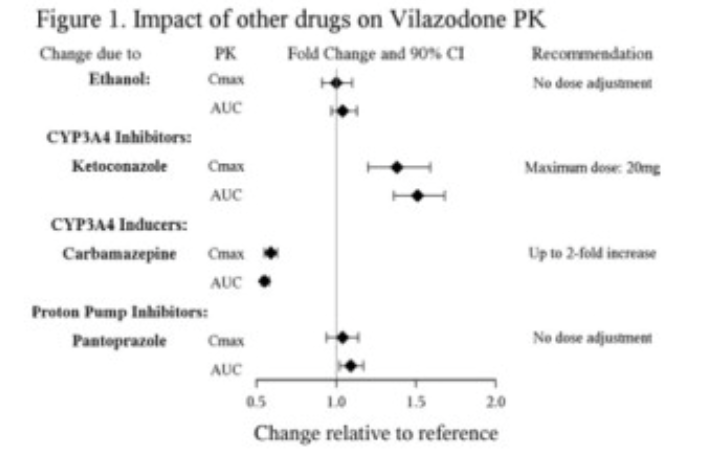
Inhibitors of CYP3A4
Metabolism by CYP3A4 is a major elimination pathway for vilazodone. Concomitant use of VIIBRYD and strong inhibitors of CYP3A4 (e.g., ketoconazole) can increase vilazodone plasma concentrations by approximately 50% (see Figure 1). The VIIBRYD dose should be reduced to 20 mg if co-administered with a strong inhibitor of CYP3A4. During co-administration with moderate inhibitors of CYP3A4 (e.g., erythromycin), the VIIBRYD dose should be reduced to 20 mg for patients with intolerable adverse events. No dose adjustment is recommended when VIIBRYD is co-administered with mild inhibitors of CYP3A4 (e.g., cimetidine) [see Dosage and Administration (2.3)].
Inducers of CYP3A4
Based on clinical response, consider increasing the dose of VIIBRYD up to 2-fold when concomitantly used with strong CYP3A4 inducers (e.g., carbamazepine) for greater than 14 days. The maximum daily dose should not exceed 80 mg. Concomitant use of VIIBRYD with strong inducers of CYP3A4 (e.g., carbamazepine) can decrease vilazodone systemic exposure by approximately 45% (see Figure 1). If CYP3A4 inducers are discontinued, reduce the VIIBRYD dose to the original level in 14 days [see Dosage and Administration (2.3)].
Inhibitors of other CYP enzymes
Concomitant administration of VIIBRYD with inhibitors of CYP2C19 and CYP2D6 is not expected to alter plasma concentrations of vilazodone. These isoforms are minor elimination pathways in the metabolism of vilazodone. In vitro studies have shown that CYP1A2, CYP2A6, CYP2C9 and CYP2E1 have minimal contribution to the metabolism of vilazodone.
Potential for Vilazodone to Affect Other Drugs
Drugs metabolized by CYP1A2, CYP2C9, CYP2D6, CYP3A4 or CYP2C19.
Co-administration of VIIBRYD with substrates for CYP1A2, CYP2C9, CYP3A4, or CYP2D6 is unlikely to result in clinically significant changes in the concentrations of the CYP substrates. A study in healthy subjects found that VIIBRYD (20 mg/day for 8-10 days) had no effect on the pharmacokinetics of caffeine, flurbiprofen, nifedipine or debrisoquine, probes for CYP1A2, CYP2C9, CYP3A4, and CYP2D6, respectively. VIIBRYD co-administration with mephenytoin to healthy subjects resulted in a small (11%) increase in mephenytoin biotransformation, suggestive of a minor induction of CYP2C19. In vitro studies have shown that VIIBRYD is a moderate inhibitor of CYP2C19 and CYP2D6.
Drugs metabolized by CYP2C8
Co-administration of VIIBRYD with a CYP2C8 substrate may lead to an increase in concentration of the other drug. In vitro studies suggest that VIIBRYD may inhibit the biotransformation of substrates of CYP2C8. The effect of VIIBRYD on CYP2C8 activity has not been tested in vivo.
Induction of CYP isoforms
VIIBRYD did not induce CYP1A1, 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, 3A4 or 3A5 in an in vitro study in cultured human hepatocytes. Chronic administration of vilazodone is unlikely to induce the metabolism of drugs metabolized by these major CYP isoforms.
Drugs Highly Bound to Plasma Protein
The interaction between vilazodone and other highly protein-bound drugs has not been evaluated. Because vilazodone is highly bound to plasma protein, administration of VIIBRYD to a patient taking another drug that is highly protein bound may cause increased free concentrations of the other dr
Triptans
There are postmarketing reports of serotonin syndrome with concomitant use of a serotonergic antidepressant and a triptan. If concomitant treatment with VIIBRYD and a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see Warnings and Precautions (5.2)].
Alcohol
As with other psychotropic medications, the use of alcohol by patients taking VIIBRYD is not recommended, because of the potential for pharmacodynamic interactions.
Use in Specific Populations
Pregnancy
Teratogenic Effects
Vilazodone caused some developmental toxicity in rats, but was not teratogenic in rats or rabbits. There are no adequate and well-controlled studies of VIIBRYD in pregnant women. When treating pregnant women with VIIBRYD, carefully consider whether the potential benefits outweigh the potential risks of treatment.
No teratogenic effects were observed when vilazodone was given to pregnant rats or rabbits during the period of organogenesis at oral doses up to 200 and 36 mg/kg/day, respectively. These doses are 48 and 17 times, in rats and rabbits, respectively, the maximum recommended human dose (MRHD) of 40 mg on a mg/m2 basis. Fetal body weight gain was reduced, and skeletal ossification was delayed in both rats and rabbits at these doses; these effects were not observed at doses up to 10 times the MRHD in rats or 4 times the MRHD in rabbits.
When vilazodone was administered to pregnant rats at an oral dose of 30 times the MRHD during the period of organogenesis and throughout pregnancy and lactation, the number of live born pups was decreased. There was an increase in early postnatal pup mortality, and among surviving pups there was decreased body weight, delayed maturation, and decreased fertility in adulthood. There was some maternal toxicity at this dose. These effects were not seen at 6 times the MRHD.
Nonteratogenic Effects
Neonates exposed to VIIBRYD and other SSRIs or serotonin and norepinephrine reuptake inhibitors (SNRIs), late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These features are consistent with either a direct toxic effect of SSRIs and SNRIs or, possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions (5.2)].
Infants exposed to SSRIs in pregnancy may have an increased risk for persistent pulmonary hypertension of the newborn (PPHN). PPHN occurs in 1-2 per 1,000 live births in the general population and is associated with substantial neonatal morbidity and mortality. Several recent epidemiologic studies suggest a positive statistical association between SSRI use (including VIIBRYD) in pregnancy and PPHN. Other studies do not show a significant statistical association.
Physicians should also note the results of a prospective longitudinal study of 201 pregnant women with a history of major depression, who were either on antidepressants or had received antidepressants less than 12 weeks prior to their last menstrual period, and were in remission. Women who discontinued antidepressant medication during pregnancy showed a significant increase in relapse of their major depression compared to those women who remained on antidepressant medication throughout pregnancy.
When treating a pregnant woman with VIIBRYD, the physician should carefully consider both the potential risks of taking an SSRI, along with the established benefits of treating depression with an antidepressant. This decision can only be made on a case by case basis.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vilazodone in women who are pregnant.
Labor and Delivery
The effect of VIIBRYD on labor and delivery in humans is unknown. VIIBRYD should be used during labor and delivery only if the potential benefit outweighs the potential risk.
Nursing Mothers
Vilazodone is excreted into the milk of lactating rats. The effect of VIIBRYD on lactation and nursing in humans is unknown. Breast feeding in women treated with VIIBRYD should be considered only if the potential benefit outweighs the potential risk to the child.
Pediatric Use
Clinical studies on the use of VIIBRYD in pediatric patients have not been conducted; therefore, the safety and effectiveness of VIIBRYD in the pediatric population have not been established. VIIBRYD is not approved for use in pediatric patients [see BOX WARNING and Warnings and Precautions (5.1)].
Geriatic Use
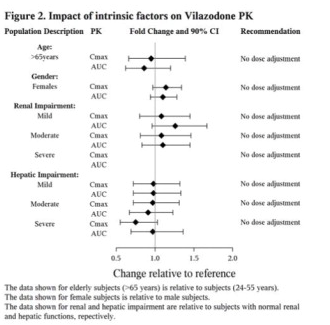
No dose adjustment is recommended on the basis of age. Results from a single-dose (20 mg) pharmacokinetic study in elderly (> 65 years-old) vs. young (24-55 years-old) subjects demonstrated that the pharmacokinetics were generally similar between the two age groups. Of the 2177 patients in clinical studies with VIIBRYD, 37 (1.7%) were 65 years of age or older, and 272 (12.5%) were 55 to 64 years of age. Greater sensitivity of some older individuals cannot be ruled out.
Serotonergic antidepressants have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event.
Gender
After adjustment for body weight, the systemic exposures between males and females are similar.
Race
There is no FDA guidance on the use of Vilazodone with respect to specific racial populations.
Renal Impairment
In mild, moderate, and severe renal impairment, no dose adjustment is necessary
Hepatic Impairment
Vilazodone is eliminated primarily by hepatic metabolism. In mild, moderate, and severe hepatic impairment, no dose adjustment is necessary (see Figure 2).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vilazodone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vilazodone in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Vilazodone Administration in the drug label.
Monitoring
There is limited information regarding Vilazodone Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Vilazodone and IV administrations.
Overdosage
There is limited information regarding Vilazodone overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Vilazodone Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Vilazodone Mechanism of Action in the drug label.
Structure
There is limited information regarding Vilazodone Structure in the drug label.
Pharmacodynamics
There is limited information regarding Vilazodone Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Vilazodone Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Vilazodone Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Vilazodone Clinical Studies in the drug label.
How Supplied
There is limited information regarding Vilazodone How Supplied in the drug label.
Storage
There is limited information regarding Vilazodone Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Vilazodone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Vilazodone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Vilazodone Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Vilazodone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Vilazodone Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Vilazodone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.