Terbutaline (oral): Difference between revisions
No edit summary |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| (20 intermediate revisions by 3 users not shown) | |||
| Line 5: | Line 5: | ||
|drugClass=[[Beta-2 adrenergic agonist]], [[bronchodilator]] and [[sympathomimetic]] | |drugClass=[[Beta-2 adrenergic agonist]], [[bronchodilator]] and [[sympathomimetic]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=[[bronchospasm]] in patients 12 years of age and older with [[asthma]] and reversible [[bronchospasm]] associated with [[bronchitis]] and [[emphysema]] | |indication=[[bronchospasm]] in patients 12 years of age and older with [[asthma]] and reversible [[bronchospasm]] associated with [[bronchitis]] and [[emphysema]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[palpitations]], [[tachyarrhythmia]], [[headache]], [[seizure]], [[tremor]] and [[nervousness]] | |adverseReactions=[[palpitations]], [[tachyarrhythmia]], [[headache]], [[seizure]], [[tremor]], and [[nervousness]] | ||
|blackBoxWarningTitle=Warning:Tocolysis | |blackBoxWarningTitle=Warning: Tocolysis | ||
|blackBoxWarningBody=Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration | |blackBoxWarningBody=Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration | ||
|fdaLIADAdult=The usual oral dose of terbutaline sulfate tablets for adults is 5 mg administered at approximately six-hour intervals, three times daily, during the hours the patient is usually awake. If side effects are particularly disturbing, the dose may be reduced to 2.5 mg three times daily, and still provide a clinically significant improvement in pulmonary function. The total dose within 24 hours should not exceed 15 mg. | |fdaLIADAdult=*The usual oral dose of terbutaline sulfate tablets for adults is 5 mg administered at approximately six-hour intervals, three times daily, during the hours the patient is usually awake. If side effects are particularly disturbing, the dose may be reduced to 2.5 mg three times daily, and still provide a clinically significant improvement in pulmonary function. The total dose within 24 hours should not exceed 15 mg. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Terbutaline in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Terbutaline in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Terbutaline in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Terbutaline (oral) in adult patients. | ||
|fdaLIADPed=Terbutaline sulfate tablets is not recommended for use in children below the age of 12 years. A dosage of 2.5 mg three times daily is recommended for children 12-15 years of age. The total dose within 24 hours should not exceed 7.5 mg. If a previously effective dosage regimen fails to provide the usual relief, medical advice should be sought immediately as this is often a sign of seriously worsening asthma that would require reassessment of therapy. | |fdaLIADPed=*Terbutaline sulfate tablets is not recommended for use in children below the age of 12 years. A dosage of 2.5 mg three times daily is recommended for children 12-15 years of age. The total dose within 24 hours should not exceed 7.5 mg. If a previously effective dosage regimen fails to provide the usual relief, medical advice should be sought immediately as this is often a sign of seriously worsening asthma that would require reassessment of therapy. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Terbutaline in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Terbutaline in pediatric patients. | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport======Prophylaxis of Exercise Induced Asthma<ref name="pmid7065478">{{cite journal| author=Sly RM, O'Brien SR| title=Effect of oral terbutaline on exercise-induced asthma. | journal=Ann Allergy | year= 1982 | volume= 48 | issue= 3 | pages= 151-5 | pmid=7065478 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7065478 }} </ref>===== | ||
|contraindications======Tocolysis===== | |contraindications======Tocolysis===== | ||
Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance [[tocolysis]]. | *Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance [[tocolysis]]. | ||
=====Hypersensitivity===== | =====Hypersensitivity===== | ||
Terbutaline sulfate is contraindicated in patients known to be hypersensitive to [[sympathomimetic amines]] or any components of this drug product. | *Terbutaline sulfate is contraindicated in patients known to be hypersensitive to [[sympathomimetic amines]] or any components of this drug product. | ||
|warnings======Deterioration of Asthma===== | |warnings======Deterioration of Asthma===== | ||
Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of terbutaline sulfate tablets, USP than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids. | *[[Asthma]] may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of terbutaline sulfate tablets, USP than usual, this may be a marker of destabilization of [[asthma]] and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for [[anti-inflammatory treatment]], e.g., [[corticosteroids]]. | ||
=====Use of Anti-Inflammatory Agents===== | =====Use of Anti-Inflammatory Agents===== | ||
The use of beta-adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids. | *The use of [[beta-adrenergic agonist]] [[bronchodilators]] alone may not be adequate to control [[asthma]] in many patients. Early consideration should be given to adding [[anti-inflammatory agents]], e.g., [[corticosteroids]]. | ||
=====Cardiovascular Effects===== | =====Cardiovascular Effects===== | ||
Terbutaline sulfate tablets, USP, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of terbutaline sulfate tablets, USP at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, terbutaline sulfate tablets, USP, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. | *Terbutaline sulfate tablets, USP, like all other [[beta-adrenergic agonists]], can produce a clinically significant cardiovascular effect in some patients as measured by [[pulse rate]], [[blood pressure]], and/or symptoms. Although such effects are uncommon after administration of terbutaline sulfate tablets, USP at recommended doses, if they occur, the drug may need to be discontinued. In addition, [[beta-agonists]] have been reported to produce electrocardiogram ([[ECG]]) changes, such as flattening of the [[T wave]], prolongation of the [[QTc interval]], and [[ST segment depression]]. The clinical significance of these findings is unknown. Therefore, terbutaline sulfate tablets, USP, like all [[sympathomimetic amines]], should be used with caution in patients with cardiovascular disorders, especially [[coronary insufficiency]], [[cardiac arrhythmias]], and [[hypertension]]. | ||
=====Seizures===== | =====Seizures===== | ||
There have been rare reports of seizures in patients receiving terbutaline; seizures did not recur in these patients after the drug was discontinued. | *There have been rare reports of [[seizures]] in patients receiving terbutaline; [[seizures]] did not recur in these patients after the drug was discontinued. | ||
|clinicalTrials=Adverse reactions observed with terbutaline sulfate tablets, are similar to those commonly seen with other sympathomimetic amines. All of these reactions are generally transient in nature and usually do not require treatment. The frequency of these side effects appears to diminish with continued therapy. | |clinicalTrials=Adverse reactions observed with terbutaline sulfate tablets, are similar to those commonly seen with other [[sympathomimetic amines]]. All of these reactions are generally transient in nature and usually do not require treatment. The frequency of these side effects appears to diminish with continued therapy. | ||
The following table lists the adverse reactions seen in 199 patients treated with terbutaline sulfate tablets during six double-blind crossover studies and four double-blind parallel studies (short- and long-term) performed in the United States. | '''The following table lists the adverse reactions seen in 199 patients treated with terbutaline sulfate tablets during six double-blind crossover studies and four double-blind parallel studies (short- and long-term) performed in the United States.''' | ||
====Adverse Reactions (Total Daily Dosage Range 5-15 mg) -Study sample size N=199==== | ====Adverse Reactions (Total Daily Dosage Range 5-15 mg) -Study sample size N=199==== | ||
=====Nervous System===== | =====Nervous System===== | ||
*[[Nervousness]] 35% | *[[Nervousness]] 35% | ||
| Line 48: | Line 46: | ||
=====Cardiovascular===== | =====Cardiovascular===== | ||
*[[Palpitations]] 5.0% | *[[Palpitations]] 5.0% | ||
*[[Tachycardia]] 3.5% | *[[Tachycardia]] 3.5% | ||
*[[Extrasystoles]] ventricular 1.5% | *[[Extrasystoles]] ventricular 1.5% | ||
[[Vasodilation]] 1.0% | *[[Vasodilation]] 1.0% | ||
=====Digestive===== | =====Digestive===== | ||
| Line 62: | Line 60: | ||
=====Skin and Appendages===== | =====Skin and Appendages===== | ||
[ | *[[Sweating]] 1.0% | ||
The following adverse effects each occurred in fewer than 1% of patients: [[ | The following adverse effects each occurred in fewer than 1% of patients: | ||
*[[Hallucinations]] | |||
*[[Rash]] | |||
*[[Paresthesia]] | |||
*[[Hypertonia]] ([[muscle cramps]]) | |||
*[[Vomiting]] | |||
There have been rare reports of elevation in liver enzymes and of [[hypersensitivity vasculitis]]. | There have been rare reports of elevation in liver enzymes and of [[hypersensitivity vasculitis]]. | ||
| | |drugInteractions=====Drug Interactions==== | ||
*The concomitant use of terbutaline sulfate tablets, USP with other [[sympathomimetic agents]] is not recommended, since the combined effect on the cardiovascular system may be deleterious to the patient. However, this does not preclude the use of an aerosol bronchodilator of the adrenergic-stimulant type for the relief of an [[acute bronchospasm]] in patients receiving chronic oral therapy with terbutaline sulfate tablets, USP. | |||
=====Monoamine Oxidase Inhibitors and Tricyclic Antidepressants===== | |||
*Terbutaline sulfate should be administered with extreme caution to patients being treated with [[monoamine oxidase inhibitors]] or [[tricyclic antidepressants]], or within 2 weeks of discontinuation of such agents, since the action of terbutaline sulfate on the vascular system may be potentiated. | |||
=====Beta-Blockers===== | |||
*[[Beta-adrenergic receptor]] blocking agents not only block the pulmonary effect of [[beta-agonists]], such as terbutaline sulfate tablets, USP, but may produce severe [[bronchospasm]] in asthmatic patients. Therefore, patients with [[asthma]] should not normally be treated with [[beta-blockers]]. However, under certain circumstances, e.g., as prophylaxis after [[myocardial infarction]], there may be no acceptable alternatives to the use of [[beta-adrenergic blocking agent]]s in patients with [[asthma]]. In this setting, cardioselective [[beta-blockers]] could be considered, although they should be administered with caution. | |||
Terbutaline sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | =====Diuretics===== | ||
|useInLaborDelivery=Because of the potential for beta-agonist interference with uterine contractility, use of terbutaline sulfate tablets | *The ECG changes and/or [[hypokalemia]] that may result from the administration of non-potassium sparing [[diuretics]] (such as [[loop diuretics]] or [[thiazide diuretics]]) can be acutely worsened by [[beta-agonists]], especially when the recommended dose of the [[beta-agonist]] is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co-administration of [[beta-agonists]] with [[non-potassium sparing diuretics]]. | ||
|FDAPregCat=C | |||
Terbutaline crosses the placenta. After single dose IV administration of terbutaline to 22 women in late pregnancy who were delivered by elective Cesarean section due to clinical reasons, umbilical blood levels of terbutaline were found to range from 11% to 48% of the maternal blood levels. | |useInPregnancyFDA=*There are no adequate and well-controlled studies of terbutaline sulfate in pregnant women. Published animal studies show that rat offspring exhibit alterations in behavior and brain development, including decreased cellular proliferation and differentiation when dams were treated subcutaneously with terbutaline during the late stage of pregnancy and lactation period. Terbutaline exposures in rat dams were approximately 6.5 times the common human dose in adults of 15 mg/day, on a mg/m2 basis. | ||
*Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance [[tocolysis]]. In particular, terbutaline sulfate should not be used for [[tocolysis]] in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient [[hyperglycemia]], [[hypokalemia]], [[cardiac arrhythmias]], [[pulmonary edema]] and [[myocardial ischemia]]. Increased fetal [[heart rate]] and neonatal [[hypoglycemia]] may occur as a result of maternal administration. | |||
|useInNursing=It is not known whether this drug is excreted in human milk. Therefore, terbutaline sulfate tablets, USP should be used during nursing only if the potential benefit justifies the possible risk to the newborn. | *In animal embryofetal development studies, no teratogenic effects were observed in offspring when pregnant rats and rabbits received terbutaline sulfate at oral doses up to 50 mg/kg/day, approximately 32 and 65 times, respectively, the maximum recommended daily oral dose for adults, on a mg/m2 basis. | ||
|useInPed=Terbutaline sulfate tablets, USP are not recommended for patients under the age of 12 years because of insufficient clinical data to establish safety and effectiveness. | *Terbutaline sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | ||
|useInLaborDelivery=*Because of the potential for [[beta-agonist]] interference with [[uterine contractility]], use of terbutaline sulfate tablets for relief of [[bronchospasm]] during labor should be restricted to those patients in whom the benefits clearly outweigh the risk. | |||
*Terbutaline crosses the [[placenta]]. After single dose IV administration of terbutaline to 22 women in late pregnancy who were delivered by elective Cesarean section due to clinical reasons, umbilical blood levels of terbutaline were found to range from 11% to 48% of the maternal blood levels. | |||
|useInNursing=*It is not known whether this drug is excreted in human milk. Therefore, terbutaline sulfate tablets, USP should be used during nursing only if the potential benefit justifies the possible risk to the newborn. | |||
|useInPed=*Terbutaline sulfate tablets, USP are not recommended for patients under the age of 12 years because of insufficient clinical data to establish safety and effectiveness. | |||
|administration=*Oral | |administration=*Oral | ||
|overdose=The median subcutaneous lethal dose of terbutaline sulfate in mature rats is approximately 165 mg/kg (approximately 90 times the maximum recommended daily oral dose for adults on a mg/m2 basis). The median subcutaneous lethal dose of terbutaline sulfate in young rats is approximately 2000 mg/kg (approximately 1100 times the maximum recommended daily oral dose for adults on a mg/m2 basis). | |overdose=*The median subcutaneous lethal dose of terbutaline sulfate in mature rats is approximately 165 mg/kg (approximately 90 times the maximum recommended daily oral dose for adults on a mg/m2 basis). The median subcutaneous lethal dose of terbutaline sulfate in young rats is approximately 2000 mg/kg (approximately 1100 times the maximum recommended daily oral dose for adults on a mg/m2 basis). | ||
*The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under adverse reactions, e.g., [[seizures]], [[angina]], [[hypertension]] or [[hypotension]], [[tachycardia]] with rates up to 200 beats per minute, [[arrhythmias]], [[nervousness]], [[headache]], [[tremor]], [[dry mouth]], [[palpitation]], [[nausea]], [[dizziness]], [[fatigue]], [[malaise]], and [[insomnia]]. [[Hypokalemia]] may also occur. | |||
*There is no specific antidote. Treatment consists of discontinuation of terbutaline sulfate tablets, USP together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce [[bronchospasm]]. There is insufficient evidence to determine if dialysis is beneficial for overdosage of terbutaline sulfate tablets. | |||
*In the alert patient who has taken excessive oral medication, the stomach should be emptied by induced emesis followed by lavage. In the unconscious patient, the airway should be secured with a cuffed endotracheal tube before lavage, and emesis should not be induced. Instillation of activated charcoal slurry may help reduce absorption of terbutaline. Adequate respiratory exchange should be maintained, and cardiac and respiratory support provided as needed. The patient should be monitored until signs and symptoms of overdosage have subsided. | |||
|drugBox={{drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 470602258 | |||
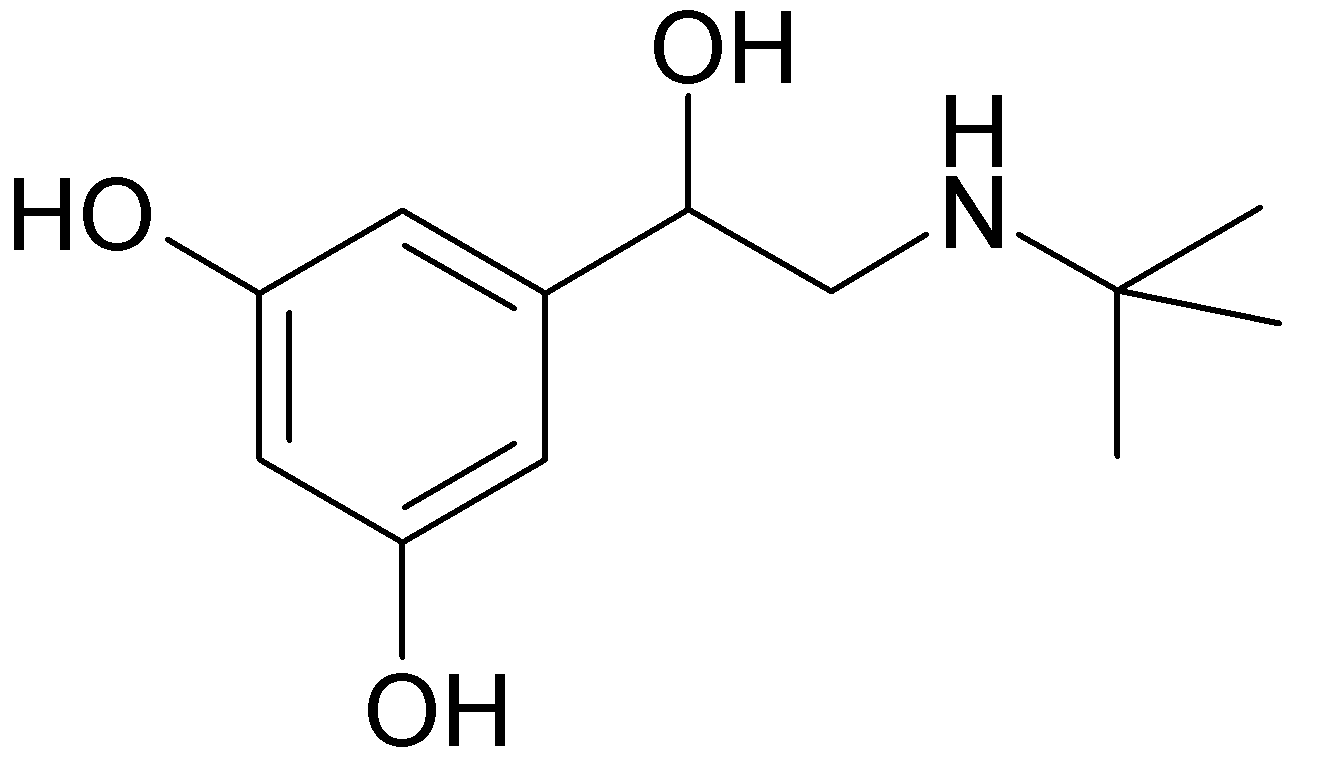
| IUPAC_name = (''RS'')-5-[2-(''tert''-butylamino)-1-hydroxyethyl]benzene-1,3-diol | |||
| image = Terbutaline.png | |||
| width = 200px | |||
| imagename = 1 : 1 mixture (racemate) | |||
| image2 = Terbutaline ball-and-stick.png | |||
| width2 = 200px | |||
| drug_name = Terbutaline | |||
<!--Clinical data--> | |||
| Drugs.com = {{drugs.com|monograph|terbutaline-sulfate}} | |||
| MedlinePlus = a682144 | |||
| pregnancy_category = C | |||
| legal_AU = | |||
| legal_CA = | |||
| legal_UK = POM | |||
| legal_US = | |||
| legal_status = | |||
| routes_of_administration = SQ, Oral, Inhaled | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = 25% | |||
| metabolism = GI tract (oral), liver; CYP450: unknown | |||
| elimination_half-life = 11-16 hours | |||
| excretion = urine 90% (60% unchanged), bile/faeces | |||
<!--Identifiers--> | |||
| | | CASNo_Ref = {{cascite|correct|CAS}} | ||
}} | | CAS_number_Ref = {{cascite|correct|??}} | ||
{{ | |||
| | |||
| | |||
| CAS_number = 23031-25-6 | | CAS_number = 23031-25-6 | ||
| ATC_prefix = R03 | | ATC_prefix = R03 | ||
| ATC_suffix = AC03 | | ATC_suffix = AC03 | ||
| ATC_supplemental = {{ATC|R03|CC03}} | | ATC_supplemental = {{ATC|R03|CC03}} | ||
| PubChem = 5403 | | PubChem = 5403 | ||
| DrugBank = | | IUPHAR_ligand = 560 | ||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00871 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 5210 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 9449 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = N8ONU3L3PG | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08570 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1760 | |||
<!--Chemical data--> | |||
| C=12 | H=19 | N=1 | O=3 | | C=12 | H=19 | N=1 | O=3 | ||
| molecular_weight = 225.284 g/mol | | molecular_weight = 225.284 g/mol | ||
| | | smiles = Oc1cc(cc(O)c1)C(O)CNC(C)(C)C | ||
| | | InChI = 1/C12H19NO3/c1-12(2,3)13-7-11(16)8-4-9(14)6-10(15)5-8/h4-6,11,13-16H,7H2,1-3H3 | ||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C12H19NO3/c1-12(2,3)13-7-11(16)8-4-9(14)6-10(15)5-8/h4-6,11,13-16H,7H2,1-3H3 | |||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChIKey = XWTYSIMOBUGWOL-UHFFFAOYSA-N | ||
| | |||
| | |||
| | |||
| | |||
| | |||
}} | }} | ||
|mechAction=*Terbutaline exerts a preferential effect on [[beta2-adrenergic receptors]]. While it is recognized that [[beta2-adrenergic receptors]] are the predominant receptors in bronchial [[smooth muscle]], data indicate that there is a population of beta2-receptors in the human heart, existing in a concentration between 10%-50%. The precise function of these receptors has not been established. In controlled clinical studies in patients given terbutaline sulfate tablets, USP orally, proportionally greater changes occurred in pulmonary function parameters than in [[heart rate]] or [[blood pressure]]. While this suggests a relative preference for the beta2-receptors in man, the usual cardiovascular effects commonly associated with other sympathomimetic agents were also observed with terbutaline sulfate tablets. | |||
|structure=Terbutaline sulfate is ±-alpha-[(tert-butylamino)methyl]-3,5-dihydroxybenzyl alcohol sulfate (2:1) (salt). The empirical formula is (C12H19NO3)2H2SO4 and the structural formula is: | |||
[[file:Terbitaline Structure.png|none|350px]] | |||
|PK======Absorption===== | |||
*Oral administration of 5-mg terbutaline sulfate tablets, USP or 5 mg terbutaline sulfate in solution in 17 healthy, adult, male subjects, resulted in mean (SD) peak plasma terbutaline concentration of 8.3 (3.9) and 8.6 (3.6) ng/mL, which were observed at median (range) times of 2 (1-3) and 1.5 (0.5-3.0) hours after dosing. The mean (SD) AUC(0-48) values were 54.6 (26.8) and 53.1 (23.5) hr∙ng/mL, and corresponded to a [[bioavailability]] of 103% for the tablet relative to the solution. | |||
=====Distribution===== | |||
*After oral administration of terbutaline, 51 to 62 mcg/kg of body weight, to 3 healthy male subjects, peak serum levels of 3.1 to 6.2 ng/mL were observed 1 to 3 hours later. In the same study, after 3 days only 30%-50% of the dose was recovered from urine and the remainder from the feces, which may indicate poor absorption. | |||
*After an oral dose to [[asthmatic]] patients, the [[elimination half-life]] of terbutaline was approximately 3.4 hours. In comparison to oral dosing, subcutaneous administration of 0.5 mg of terbutaline sulfate to 17 healthy, adult, male subjects resulted in a mean (SD) peak plasma terbutaline concentration of 9.6 (3.6) ng/mL, which was observed at a median (range) time of 0.5 (0.08-1.0) hours after dosing. The mean (SD) AUC (0-48) and total body clearance values were 29.4 (14.2) hr∙ng/mL, and 311 (112) mL/min, respectively. The terminal half-life was determined in 9 of the 17 subjects and had a mean (SD) of 5.7 (2.0) hours. | |||
== | =====Excretion===== | ||
*About 90% of the drug was excreted in the urine at 96 hours after subcutaneous administration, with about 60% of this being unchanged drug. The sulfate conjugate is a major metabolite of terbutaline, and urinary excretion is the primary route of elimination. | |||
== | *There are no reports of any clinical pharmacokinetic studies investigating dose proportionality, effect of food, or special population studies with terbutaline. | ||
|howSupplied=[[file:Terbutaline How Supplied.png|none|550px]] | |||
|storage=*Store at controlled room temperature 15° to 30°C (59° to 86°F). Dispense in tightly-closed, light-resistant container (USP). | |||
|packLabel=[[file:FDA Label1.png|none|500px]] | |||
[[file:Terbitalinetable2.png|none|500px]] | |||
|alcohol=Alcohol-Terbutaline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=*[[Brethine]] | |||
*[[Bricanyl]] | |||
*[[Brethaire]] | |||
}} | |||
{{LabelImage | |||
|fileName=Terbutaline 2.5mg.png | |||
}} | |||
{{LabelImage | |||
|fileName=Terbutaline 5.0 mg.png | |||
}} | |||
[[ | |||
[[ | |||
[[ | |||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
Latest revision as of 11:20, 25 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning: Tocolysis
See full prescribing information for complete Boxed Warning.
Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration
|
Overview
Terbutaline (oral) is a Beta-2 adrenergic agonist, bronchodilator and sympathomimetic that is FDA approved for the treatment of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and emphysema. There is a Black Box Warning for this drug as shown here. Common adverse reactions include palpitations, tachyarrhythmia, headache, seizure, tremor, and nervousness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- The usual oral dose of terbutaline sulfate tablets for adults is 5 mg administered at approximately six-hour intervals, three times daily, during the hours the patient is usually awake. If side effects are particularly disturbing, the dose may be reduced to 2.5 mg three times daily, and still provide a clinically significant improvement in pulmonary function. The total dose within 24 hours should not exceed 15 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terbutaline in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Terbutaline (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Terbutaline sulfate tablets is not recommended for use in children below the age of 12 years. A dosage of 2.5 mg three times daily is recommended for children 12-15 years of age. The total dose within 24 hours should not exceed 7.5 mg. If a previously effective dosage regimen fails to provide the usual relief, medical advice should be sought immediately as this is often a sign of seriously worsening asthma that would require reassessment of therapy.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terbutaline in pediatric patients.
Non–Guideline-Supported Use
Prophylaxis of Exercise Induced Asthma[1]
Contraindications
Tocolysis
- Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis.
Hypersensitivity
- Terbutaline sulfate is contraindicated in patients known to be hypersensitive to sympathomimetic amines or any components of this drug product.
Warnings
|
Warning: Tocolysis
See full prescribing information for complete Boxed Warning.
Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration
|
Deterioration of Asthma
- Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of terbutaline sulfate tablets, USP than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
Use of Anti-Inflammatory Agents
- The use of beta-adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids.
Cardiovascular Effects
- Terbutaline sulfate tablets, USP, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of terbutaline sulfate tablets, USP at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, terbutaline sulfate tablets, USP, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Seizures
- There have been rare reports of seizures in patients receiving terbutaline; seizures did not recur in these patients after the drug was discontinued.
Adverse Reactions
Clinical Trials Experience
Adverse reactions observed with terbutaline sulfate tablets, are similar to those commonly seen with other sympathomimetic amines. All of these reactions are generally transient in nature and usually do not require treatment. The frequency of these side effects appears to diminish with continued therapy.
The following table lists the adverse reactions seen in 199 patients treated with terbutaline sulfate tablets during six double-blind crossover studies and four double-blind parallel studies (short- and long-term) performed in the United States.
Adverse Reactions (Total Daily Dosage Range 5-15 mg) -Study sample size N=199
Nervous System
- Nervousness 35%
- Tremor 15%
- Somnolence 5.5%
- Dizziness 3.5%
- Anxiety 1.0%
- Insomnia 1-5%
Cardiovascular
- Palpitations 5.0%
- Tachycardia 3.5%
- Extrasystoles ventricular 1.5%
- Vasodilation 1.0%
Digestive
Body as a Whole
Skin and Appendages
- Sweating 1.0%
The following adverse effects each occurred in fewer than 1% of patients:
There have been rare reports of elevation in liver enzymes and of hypersensitivity vasculitis.
Postmarketing Experience
There is limited information regarding Terbutaline (oral) Postmarketing Experience in the drug label.
Drug Interactions
Drug Interactions
- The concomitant use of terbutaline sulfate tablets, USP with other sympathomimetic agents is not recommended, since the combined effect on the cardiovascular system may be deleterious to the patient. However, this does not preclude the use of an aerosol bronchodilator of the adrenergic-stimulant type for the relief of an acute bronchospasm in patients receiving chronic oral therapy with terbutaline sulfate tablets, USP.
Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
- Terbutaline sulfate should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, since the action of terbutaline sulfate on the vascular system may be potentiated.
Beta-Blockers
- Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-agonists, such as terbutaline sulfate tablets, USP, but may produce severe bronchospasm in asthmatic patients. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
Diuretics
- The ECG changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop diuretics or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co-administration of beta-agonists with non-potassium sparing diuretics.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of terbutaline sulfate in pregnant women. Published animal studies show that rat offspring exhibit alterations in behavior and brain development, including decreased cellular proliferation and differentiation when dams were treated subcutaneously with terbutaline during the late stage of pregnancy and lactation period. Terbutaline exposures in rat dams were approximately 6.5 times the common human dose in adults of 15 mg/day, on a mg/m2 basis.
- Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline sulfate should not be used for tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration.
- In animal embryofetal development studies, no teratogenic effects were observed in offspring when pregnant rats and rabbits received terbutaline sulfate at oral doses up to 50 mg/kg/day, approximately 32 and 65 times, respectively, the maximum recommended daily oral dose for adults, on a mg/m2 basis.
- Terbutaline sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Terbutaline (oral) in women who are pregnant.
Labor and Delivery
- Because of the potential for beta-agonist interference with uterine contractility, use of terbutaline sulfate tablets for relief of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risk.
- Terbutaline crosses the placenta. After single dose IV administration of terbutaline to 22 women in late pregnancy who were delivered by elective Cesarean section due to clinical reasons, umbilical blood levels of terbutaline were found to range from 11% to 48% of the maternal blood levels.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Therefore, terbutaline sulfate tablets, USP should be used during nursing only if the potential benefit justifies the possible risk to the newborn.
Pediatric Use
- Terbutaline sulfate tablets, USP are not recommended for patients under the age of 12 years because of insufficient clinical data to establish safety and effectiveness.
Geriatic Use
There is no FDA guidance on the use of Terbutaline (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Terbutaline (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Terbutaline (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Terbutaline (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Terbutaline (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Terbutaline (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Terbutaline (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Terbutaline (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Terbutaline (oral) and IV administrations.
Overdosage
- The median subcutaneous lethal dose of terbutaline sulfate in mature rats is approximately 165 mg/kg (approximately 90 times the maximum recommended daily oral dose for adults on a mg/m2 basis). The median subcutaneous lethal dose of terbutaline sulfate in young rats is approximately 2000 mg/kg (approximately 1100 times the maximum recommended daily oral dose for adults on a mg/m2 basis).
- The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under adverse reactions, e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats per minute, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and insomnia. Hypokalemia may also occur.
- There is no specific antidote. Treatment consists of discontinuation of terbutaline sulfate tablets, USP together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of terbutaline sulfate tablets.
- In the alert patient who has taken excessive oral medication, the stomach should be emptied by induced emesis followed by lavage. In the unconscious patient, the airway should be secured with a cuffed endotracheal tube before lavage, and emesis should not be induced. Instillation of activated charcoal slurry may help reduce absorption of terbutaline. Adequate respiratory exchange should be maintained, and cardiac and respiratory support provided as needed. The patient should be monitored until signs and symptoms of overdosage have subsided.
Pharmacology
| |
| |
1 : 1 mixture (racemate)Terbutaline
| |
| Systematic (IUPAC) name | |
| (RS)-5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diol | |
| Identifiers | |
| CAS number | |
| ATC code | R03 R03CC03 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 225.284 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 25% |
| Metabolism | GI tract (oral), liver; CYP450: unknown |
| Half life | 11-16 hours |
| Excretion | urine 90% (60% unchanged), bile/faeces |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status | |
| Routes | SQ, Oral, Inhaled |
Mechanism of Action
- Terbutaline exerts a preferential effect on beta2-adrenergic receptors. While it is recognized that beta2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta2-receptors in the human heart, existing in a concentration between 10%-50%. The precise function of these receptors has not been established. In controlled clinical studies in patients given terbutaline sulfate tablets, USP orally, proportionally greater changes occurred in pulmonary function parameters than in heart rate or blood pressure. While this suggests a relative preference for the beta2-receptors in man, the usual cardiovascular effects commonly associated with other sympathomimetic agents were also observed with terbutaline sulfate tablets.
Structure
Terbutaline sulfate is ±-alpha-[(tert-butylamino)methyl]-3,5-dihydroxybenzyl alcohol sulfate (2:1) (salt). The empirical formula is (C12H19NO3)2H2SO4 and the structural formula is:

Pharmacodynamics
There is limited information regarding Terbutaline (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- Oral administration of 5-mg terbutaline sulfate tablets, USP or 5 mg terbutaline sulfate in solution in 17 healthy, adult, male subjects, resulted in mean (SD) peak plasma terbutaline concentration of 8.3 (3.9) and 8.6 (3.6) ng/mL, which were observed at median (range) times of 2 (1-3) and 1.5 (0.5-3.0) hours after dosing. The mean (SD) AUC(0-48) values were 54.6 (26.8) and 53.1 (23.5) hr∙ng/mL, and corresponded to a bioavailability of 103% for the tablet relative to the solution.
Distribution
- After oral administration of terbutaline, 51 to 62 mcg/kg of body weight, to 3 healthy male subjects, peak serum levels of 3.1 to 6.2 ng/mL were observed 1 to 3 hours later. In the same study, after 3 days only 30%-50% of the dose was recovered from urine and the remainder from the feces, which may indicate poor absorption.
- After an oral dose to asthmatic patients, the elimination half-life of terbutaline was approximately 3.4 hours. In comparison to oral dosing, subcutaneous administration of 0.5 mg of terbutaline sulfate to 17 healthy, adult, male subjects resulted in a mean (SD) peak plasma terbutaline concentration of 9.6 (3.6) ng/mL, which was observed at a median (range) time of 0.5 (0.08-1.0) hours after dosing. The mean (SD) AUC (0-48) and total body clearance values were 29.4 (14.2) hr∙ng/mL, and 311 (112) mL/min, respectively. The terminal half-life was determined in 9 of the 17 subjects and had a mean (SD) of 5.7 (2.0) hours.
Excretion
- About 90% of the drug was excreted in the urine at 96 hours after subcutaneous administration, with about 60% of this being unchanged drug. The sulfate conjugate is a major metabolite of terbutaline, and urinary excretion is the primary route of elimination.
- There are no reports of any clinical pharmacokinetic studies investigating dose proportionality, effect of food, or special population studies with terbutaline.
Nonclinical Toxicology
There is limited information regarding Terbutaline (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Terbutaline (oral) Clinical Studies in the drug label.
How Supplied

Storage
- Store at controlled room temperature 15° to 30°C (59° to 86°F). Dispense in tightly-closed, light-resistant container (USP).
Images
Drug Images
{{#ask: Page Name::Terbutaline (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
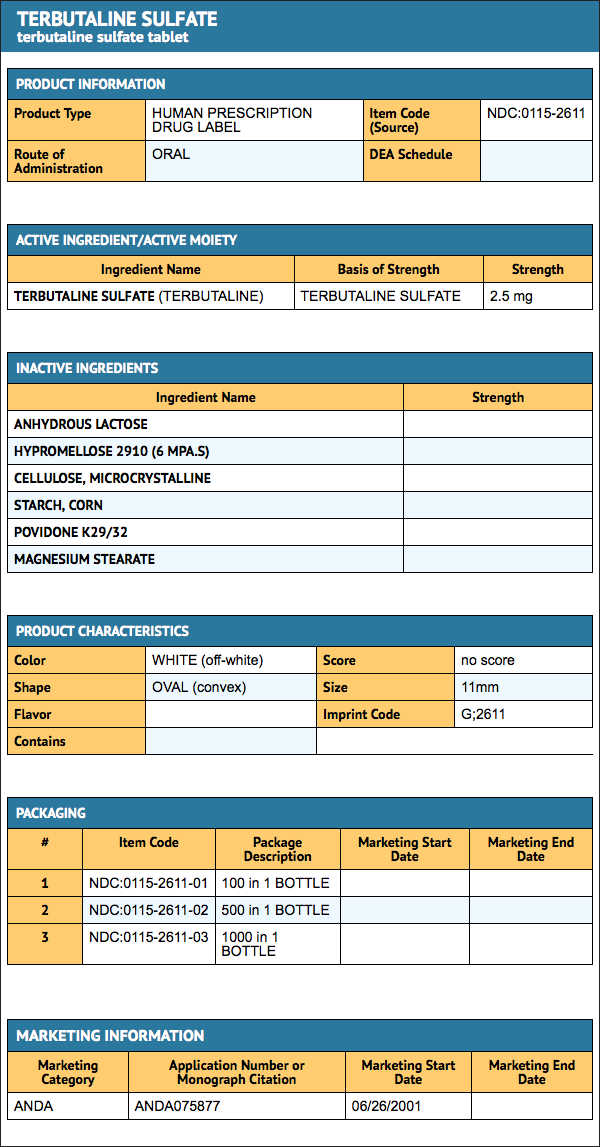

{{#ask: Label Page::Terbutaline (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Terbutaline (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Terbutaline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Terbutaline (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Sly RM, O'Brien SR (1982). "Effect of oral terbutaline on exercise-induced asthma". Ann Allergy. 48 (3): 151–5. PMID 7065478.
{{#subobject:
|Label Page=Terbutaline (oral) |Label Name=Terbutaline 2.5mg.png
}}
{{#subobject:
|Label Page=Terbutaline (oral) |Label Name=Terbutaline 5.0 mg.png
}}