Sirolimus: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
* In this and another study in de novo liver transplant patients, the use of Rapamune in combination with cyclosporine or tacrolimus was associated with an increase in HAT; most cases of HAT occurred within 30 days post-transplantation and most led to graft loss or death. | * In this and another study in de novo liver transplant patients, the use of Rapamune in combination with cyclosporine or tacrolimus was associated with an increase in HAT; most cases of HAT occurred within 30 days post-transplantation and most led to graft loss or death. | ||
LUNG TRANSPLANTATION– BRONCHIAL ANASTOMOTIC DEHISCENCE | LUNG TRANSPLANTATION– BRONCHIAL ANASTOMOTIC DEHISCENCE | ||
* Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when | * Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when sirolimus has been used as part of an immunosuppressive regimen. | ||
|fdaLIADAdult======Prophylaxis of Organ Rejection in Renal Transplantation===== | |fdaLIADAdult======Prophylaxis of Organ Rejection in Renal Transplantation===== | ||
* | * Sirolimus is indicated for the prophylaxis of organ rejection in patients aged 13 years or older receiving renal transplants. Therapeutic drug monitoring is recommended for all patients receiving sirolimus. | ||
* In patients at low- to moderate-immunologic risk, it is recommended that | * In patients at low- to moderate-immunologic risk, it is recommended that sirolimus be used initially in a regimen with cyclosporine and corticosteroids; cyclosporine should be withdrawn 2 to 4 months after transplantation. | ||
* In patients at high-immunologic risk (defined as Black recipients and/or repeat renal transplant recipients who lost a previous allograft for immunologic reason and/or patients with high panel-reactive antibodies [PRA; peak PRA level > 80%]), it is recommended that | * In patients at high-immunologic risk (defined as Black recipients and/or repeat renal transplant recipients who lost a previous allograft for immunologic reason and/or patients with high panel-reactive antibodies [PRA; peak PRA level > 80%]), it is recommended that sirolimus be used in combination with cyclosporine and corticosteroids for the first year following transplantation. | ||
=====Limitations of Use===== | =====Limitations of Use===== | ||
* Cyclosporine withdrawal has not been studied in patients with Banff Grade 3 acute rejection or vascular rejection prior to cyclosporine withdrawal, those who are dialysis-dependent, those with serum creatinine > 4.5 mg/dL, Black patients, patients of multi-organ transplants, secondary transplants, or those with high levels of panel-reactive antibodies. | * Cyclosporine withdrawal has not been studied in patients with Banff Grade 3 acute rejection or vascular rejection prior to cyclosporine withdrawal, those who are dialysis-dependent, those with serum creatinine > 4.5 mg/dL, Black patients, patients of multi-organ transplants, secondary transplants, or those with high levels of panel-reactive antibodies. | ||
* In patients at high-immunologic risk, the safety and efficacy of | * In patients at high-immunologic risk, the safety and efficacy of sirolimus used in combination with cyclosporine and corticosteroids has not been studied beyond one year; therefore after the first 12 months following transplantation, any adjustments to the immunosuppressive regimen should be considered on the basis of the clinical status of the patient. | ||
* In pediatric patients, the safety and efficacy of | * In pediatric patients, the safety and efficacy of sirolimus have not been established in patients < 13 years old, or in pediatric (< 18 years) renal transplant patients considered at high-immunologic risk. | ||
* The safety and efficacy of de novo use of | * The safety and efficacy of de novo use of sirolimus without cyclosporine have not been established in renal transplant patients. | ||
* The safety and efficacy of conversion from calcineurin inhibitors to Rapamune in maintenance renal transplant patients have not been established. | * The safety and efficacy of conversion from calcineurin inhibitors to Rapamune in maintenance renal transplant patients have not been established. | ||
* | * Sirolimus is to be administered orally once daily, consistently with or without food. | ||
* Tablets should not be crushed, chewed or split. Patients unable to take the tablets should be prescribed the solution and instructed in its use. | * Tablets should not be crushed, chewed or split. Patients unable to take the tablets should be prescribed the solution and instructed in its use. | ||
* The initial dose of | * The initial dose of sirolimus should be administered as soon as possible after transplantation. It is recommended that sirolimus be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and or/cyclosporine capsules (MODIFIED). | ||
* Frequent | * Frequent sirolimus dose adjustments based on non-steady-state sirolimus concentrations can lead to overdosing or underdosing because sirolimus has a long half-life. | ||
* Once | * Once sirolimus maintenance dose is adjusted, patients should continue on the new maintenance dose for at least 7 to 14 days before further dosage adjustment with concentration monitoring. In most patients, dose adjustments can be based on simple proportion: new sirolimus dose = current dose x (target concentration/current concentration). | ||
* A loading dose should be considered in addition to a new maintenance dose when it is necessary to increase sirolimus trough concentrations: | * A loading dose should be considered in addition to a new maintenance dose when it is necessary to increase sirolimus trough concentrations: sirolimus loading dose = 3 x (new maintenance dose - current maintenance dose). | ||
* The maximum | * The maximum sirolimus dose administered on any day should not exceed 40 mg. If an estimated daily dose exceeds 40 mg due to the addition of a loading dose, the loading dose should be administered over 2 days. | ||
* Sirolimus trough concentrations should be monitored at least 3 to 4 days after a loading dose(s). | * Sirolimus trough concentrations should be monitored at least 3 to 4 days after a loading dose(s). | ||
* Two milligrams (2 mg) of | * Two milligrams (2 mg) of Sirolimus Oral Solution have been demonstrated to be clinically equivalent to 2 mg sirolimus Tablets; hence, are interchangeable on a mg-to-mg basis. | ||
* However, it is not known if higher doses of | * However, it is not known if higher doses of sirolimus Oral Solution are clinically equivalent to higher doses of sirolimus Tablets on a mg‑to‑mg basis. | ||
====Patients at Low- to Moderate-Immunologic Risk==== | ====Patients at Low- to Moderate-Immunologic Risk==== | ||
===== | =====Sirolimus and Cyclosporine Combination Therapy===== | ||
* For de novo renal transplant patients, it is recommended that | * For de novo renal transplant patients, it is recommended that sirolimus Oral Solution and Tablets be used initially in a regimen with cyclosporine and corticosteroids. | ||
* A loading dose of | * A loading dose of sirolimus equivalent to 3 times the maintenance dose should be given, i.e. a daily maintenance dose of 2 mg should be preceded with a loading dose of 6 mg. Therapeutic drug monitoring should be used to maintain sirolimus drug concentrations within the target-range. | ||
===== | =====Sirolimus Following Cyclosporine Withdrawal===== | ||
* At 2 to 4 months following transplantation, cyclosporine should be progressively discontinued over 4 to 8 weeks, and the | * At 2 to 4 months following transplantation, cyclosporine should be progressively discontinued over 4 to 8 weeks, and the sirolimus dose should be adjusted to obtain sirolimus whole blood trough concentrations within the target-range. | ||
* Because cyclosporine inhibits the metabolism and transport of sirolimus, sirolimus concentrations may decrease when cyclosporine is discontinued, unless the | * Because cyclosporine inhibits the metabolism and transport of sirolimus, sirolimus concentrations may decrease when cyclosporine is discontinued, unless the sirolimus dose is increased. | ||
=====Patients at High-Immunologic Risk===== | =====Patients at High-Immunologic Risk===== | ||
* In patients with high-immunologic risk, it is recommended that | * In patients with high-immunologic risk, it is recommended that sirolimus be used in combination with cyclosporine and corticosteroids for the first 12 months following transplantation. * The safety and efficacy of this combination in high-immunologic risk patients has not been studied beyond the first 12 months. Therefore, after the first 12 months following transplantation, any adjustments to the immunosuppressive regimen should be considered on the basis of the clinical status of the patient. | ||
* For patients receiving | * For patients receiving sirolimus with cyclosporine, sirolimus therapy should be initiated with a loading dose of up to 15 mg on day 1 post-transplantation. Beginning on day 2, an initial maintenance dose of 5 mg/day should be given. A trough level should be obtained between days 5 and 7, and the daily dose of sirolimus should thereafter be adjusted. | ||
* The starting dose of cyclosporine should be up to 7 mg/kg/day in divided doses and the dose should subsequently be adjusted to achieve target whole blood trough concentrations. Prednisone should be administered at a minimum of 5 mg/day. | * The starting dose of cyclosporine should be up to 7 mg/kg/day in divided doses and the dose should subsequently be adjusted to achieve target whole blood trough concentrations. Prednisone should be administered at a minimum of 5 mg/day. | ||
* Antibody induction therapy may be used. | * Antibody induction therapy may be used. | ||
=====Therapeutic Drug Monitoring===== | =====Therapeutic Drug Monitoring===== | ||
* Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the | * Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the sirolimus dosage form is made, and during concurrent administration of strong CYP3A4 inducers and inhibitors. | ||
* Therapeutic drug monitoring should not be the sole basis for adjusting | * Therapeutic drug monitoring should not be the sole basis for adjusting sirolimus therapy. Careful attention should be made to clinical signs/symptoms, tissue biopsy findings, and laboratory parameters. | ||
* When used in combination with cyclosporine, sirolimus trough concentrations should be maintained within the target-range. | * When used in combination with cyclosporine, sirolimus trough concentrations should be maintained within the target-range. | ||
* Following cyclosporine withdrawal in transplant patients at low- to moderate-immunologic risk, the target sirolimus trough concentrations should be 16 to 24 ng/mL for the first year following transplantation. Thereafter, the target sirolimus concentrations should be 12 to 20 ng/mL. | * Following cyclosporine withdrawal in transplant patients at low- to moderate-immunologic risk, the target sirolimus trough concentrations should be 16 to 24 ng/mL for the first year following transplantation. Thereafter, the target sirolimus concentrations should be 12 to 20 ng/mL. | ||
| Line 60: | Line 60: | ||
* The initial dosage in patients ≥ 13 years who weigh less than 40 kg should be adjusted, based on body surface area, to 1 mg/m2/day. The loading dose should be 3 mg/m2. | * The initial dosage in patients ≥ 13 years who weigh less than 40 kg should be adjusted, based on body surface area, to 1 mg/m2/day. The loading dose should be 3 mg/m2. | ||
=====Patients with Hepatic Impairment===== | =====Patients with Hepatic Impairment===== | ||
* It is recommended that the maintenance dose of | * It is recommended that the maintenance dose of sirolimus be reduced by approximately one third in patients with mild or moderate hepatic impairment and by approximately one half in patients with severe hepatic impairment. It is not necessary to modify the sirolimus loading dose. | ||
=====Patients with Renal Impairment===== | =====Patients with Renal Impairment===== | ||
* Dosage adjustment is not needed in patients with impaired renal function. | * Dosage adjustment is not needed in patients with impaired renal function. | ||
=====Instructions for Dilution and Administration of | =====Instructions for Dilution and Administration of sirolimus Oral Solution===== | ||
* The amber oral dose syringe should be used to withdraw the prescribed amount of | * The amber oral dose syringe should be used to withdraw the prescribed amount of sirolimus Oral Solution from the bottle. Empty the correct amount of sirolimus from the syringe into only a glass or plastic container holding at least two (2) ounces (1/4 cup, 60 mL) of water or orange juice. No other liquids, including grapefruit juice, should be used for dilution. Stir vigorously and drink at once. Refill the container with an additional volume [minimum of four (4) ounces (1/2 cup, 120 mL)] of water or orange juice, stir vigorously, and drink at once. | ||
* | * Sirolimus Oral Solution contains polysorbate 80, which is known to increase the rate of di‑(2‑ethylhexyl)phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of sirolimus Oral Solution. It is important that these recommendations be followed closely. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 82: | Line 82: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=* Sirolimus is contraindicated in patients with a hypersensitivity to sirolimus. | ||
|warnings======Increased Susceptibility to Infection and the Possible Development of Lymphoma===== | |warnings======Increased Susceptibility to Infection and the Possible Development of Lymphoma===== | ||
* Increased susceptibility to infection and the possible development of lymphoma and other malignancies, particularly of the skin, may result from immunosuppression. The rates of lymphoma/lymphoproliferative disease observed in Studies 1 and 2 were 0.7-3.2% (for | * Increased susceptibility to infection and the possible development of lymphoma and other malignancies, particularly of the skin, may result from immunosuppression. The rates of lymphoma/lymphoproliferative disease observed in Studies 1 and 2 were 0.7-3.2% (for sirolimus-treated patients) versus 0.6-0.8% (azathioprine and placebo control). | ||
* Oversuppression of the immune system can also increasesusceptibility to infection, including opportunistic infections such as tuberculosis, fatal infections, and sepsis. Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should use | * Oversuppression of the immune system can also increasesusceptibility to infection, including opportunistic infections such as tuberculosis, fatal infections, and sepsis. Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should use sirolimus. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient. | ||
=====Liver Transplantation – Excess Mortality, Graft Loss, and Hepatic Artery Thrombosis (HAT)===== | =====Liver Transplantation – Excess Mortality, Graft Loss, and Hepatic Artery Thrombosis (HAT)===== | ||
* The use of | * The use of sirolimus in combination with tacrolimus was associated with excess mortality and graft loss in a study in de novo liver transplant patients (22% in combination versus 9% on tacrolimus alone). Many of these patients had evidence of infection at or near the time of death. | ||
* In this and another study in de novo liver transplant patients, the use of | * In this and another study in de novo liver transplant patients, the use of sirolimus in combination with cyclosporine or tacrolimus was associated with an increase in HAT (7% in combination versus 2% in the control arm); most cases of HAT occurred within 30 days post-transplantation, and most led to graft loss or death. | ||
* The safety and efficacy of | * The safety and efficacy of sirolimus as immunosuppressive therapy have not been established in liver transplant patients; therefore, such use is not recommended. | ||
=====Lung Transplantation – Bronchial Anastomotic Dehiscence===== | =====Lung Transplantation – Bronchial Anastomotic Dehiscence===== | ||
* Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when | * Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when sirolimus has been used as part of an immunosuppressive regimen. | ||
* The safety and efficacy of | * The safety and efficacy of sirolimus as immunosuppressive therapy have not been established in lung transplant patients; therefore, such use is not recommended. | ||
=====Hypersensitivity Reactions===== | =====Hypersensitivity Reactions===== | ||
* Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis and hypersensitivity vasculitis, have been associated with the administration of | * Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis and hypersensitivity vasculitis, have been associated with the administration of sirolimus. | ||
=====Angioedema===== | =====Angioedema===== | ||
* | * Sirolimus has been associated with the development of angioedema. The concomitant use of sirolimus with other drugs known to cause angioedema, such as ACE-inhibitors, may increase the risk of developing angioedema. | ||
=====Fluid Accumulation and Wound Healing===== | =====Fluid Accumulation and Wound Healing===== | ||
* There have been reports of impaired or delayed wound healing in patients receiving | * There have been reports of impaired or delayed wound healing in patients receiving sirolimus, including lymphocele and wound dehiscence. | ||
* mTOR inhibitors such as sirolimus have been shown in vitro to inhibit production of certain growth factors that may affect angiogenesis, fibroblast proliferation, and vascular permeability. Lymphocele, a known surgical complication of renal transplantation, occurred significantly more often in a dose-related fashion in patients treated with | * mTOR inhibitors such as sirolimus have been shown in vitro to inhibit production of certain growth factors that may affect angiogenesis, fibroblast proliferation, and vascular permeability. Lymphocele, a known surgical complication of renal transplantation, occurred significantly more often in a dose-related fashion in patients treated with sirolimus. | ||
* Appropriate measures should be considered to minimize such complications. Patients with a body mass index (BMI) greater than 30 kg/m2 may be at increased risk of abnormal wound healing based on data from the medical literature. | * Appropriate measures should be considered to minimize such complications. Patients with a body mass index (BMI) greater than 30 kg/m2 may be at increased risk of abnormal wound healing based on data from the medical literature. | ||
* There have also been reports of fluid accumulation, including peripheral edema, lymphedema, pleural effusion and pericardial effusions (including hemodynamically significant effusions and tamponade requiring intervention in children and adults), in patients receiving | * There have also been reports of fluid accumulation, including peripheral edema, lymphedema, pleural effusion and pericardial effusions (including hemodynamically significant effusions and tamponade requiring intervention in children and adults), in patients receiving sirolimus. | ||
=====Hyperlipidemia===== | =====Hyperlipidemia===== | ||
* Increased serum cholesterol and triglycerides requiring treatment occurred more frequently in patients treated with | * Increased serum cholesterol and triglycerides requiring treatment occurred more frequently in patients treated with sirolimus compared with azathioprine or placebo controls in Studies 1 and 2. | ||
* There were increased incidences of hypercholesterolemia (43-46%) and/or hypertriglyceridemia (45-57%) in patients receiving | * There were increased incidences of hypercholesterolemia (43-46%) and/or hypertriglyceridemia (45-57%) in patients receiving sirolimus compared with placebo controls (each 23%). The risk/benefit should be carefully considered in patients with established hyperlipidemia before initiating an immunosuppressive regimen including sirolimus. | ||
* Any patient who is administered | * Any patient who is administered sirolimus should be monitored for hyperlipidemia. If detected, interventions such as diet, exercise, and lipid-lowering agents should be initiated as outlined by the National Cholesterol Education Program guidelines. | ||
* In clinical trials, the concomitant administration of | * In clinical trials, the concomitant administration of sirolimus and HMG-CoA reductase inhibitors and/or fibrates appeared to be well-tolerated. | ||
* During | * During sirolimus therapy with cyclosporine, patients administered an HMG-CoA reductase inhibitor and/or fibrate should be monitored for the possible development of rhabdomyolysis and other adverse effects, as described in the respective labeling for these agents. | ||
=====Renal Function===== | =====Renal Function===== | ||
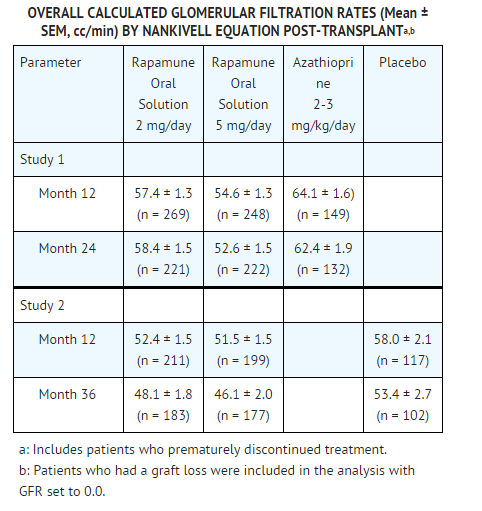
* Renal function should be closely monitored during the co-administration of | * Renal function should be closely monitored during the co-administration of sirolimus with cyclosporine, because long-term administration of the combination has been associated with deterioration of renal function. Patients treated with cyclosporine and sirolimus were noted to have higher serum creatinine levels and lower glomerular filtration rates compared with patients treated with cyclosporine and placebo or azathioprine controls (Studies 1 and 2). The rate of decline in renal function in these studies was greater in patients receiving sirolimus and cyclosporine compared with control therapies. | ||
* Appropriate adjustment of the immunosuppressive regimen, including discontinuation of | * Appropriate adjustment of the immunosuppressive regimen, including discontinuation of sirolimus and/or cyclosporine, should be considered in patients with elevated or increasing serum creatinine levels. In patients at low- to moderate-immunologic risk, continuation of combination therapy with cyclosporine beyond 4 months following transplantation should only be considered when the benefits outweigh the risks of this combination for the individual patients. Caution should be exercised when using agents (e.g., aminoglycosides and amphotericin B) that are known to have a deleterious effect on renal function. | ||
* In patients with delayed graft function, | * In patients with delayed graft function, sirolimus may delay recovery of renal function. | ||
=====Proteinuria===== | =====Proteinuria===== | ||
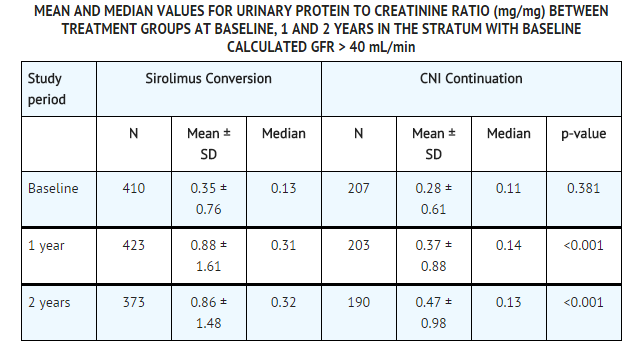
* Periodic quantitative monitoring of urinary protein excretion is recommended. In a study evaluating conversion from calcineurin inhibitors (CNI) to | * Periodic quantitative monitoring of urinary protein excretion is recommended. In a study evaluating conversion from calcineurin inhibitors (CNI) to sirolimus in maintenance renal transplant patients 6-120 months post-transplant, increased urinary protein excretion was commonly observed from 6 through 24 months after conversion to sirolimus compared with CNI continuation. | ||
* Patients with the greatest amount of urinary protein excretion prior to | * Patients with the greatest amount of urinary protein excretion prior to sirolimus conversion were those whose protein excretion increased the most after conversion.New onset nephrosis (nephrotic syndrome) was also reported as a treatment-emergent adverse event in 2.2% of the sirolimus conversion group patients in comparison to 0.4% in the CNI continuation group of patients. Nephrotic range proteinuria (defined as urinary protein to creatinine ratio > 3.5) was also reported in 9.2% in the sirolimus conversion group of patients in comparison to 3.7% in the CNI continuation group of patients. In some patients, reduction in the degree of urinary protein excretion was observed for individual patients following discontinuation of sirolimus. | ||
* The safety and efficacy of conversion from calcineurin inhibitors to | * The safety and efficacy of conversion from calcineurin inhibitors to sirolimus in maintenance renal transplant patients have not been established. | ||
=====Interstitial Lung Disease===== | =====Interstitial Lung Disease===== | ||
* Cases of interstitial lung disease (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving immunosuppressive regimens including | * Cases of interstitial lung disease (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving immunosuppressive regimens including sirolimus. In some cases, the interstitial lung disease has resolved upon discontinuation or dose reduction of sirolimus. The risk may be increased as the trough sirolimus concentration increases. | ||
=====De Novo Use Without Cyclosporine===== | =====De Novo Use Without Cyclosporine===== | ||
* The safety and efficacy of de novo use of | * The safety and efficacy of de novo use of sirolimus without cyclosporine is not established in renal transplant patients. In a multicenter clinical study, de novo renal transplant patients treated with sirolimus, mycophenolate mofetil (MMF), steroids, and an IL-2 receptor antagonist had significantly higher acute rejection rates and numerically higher death rates compared to patients treated with cyclosporine, MMF, steroids, and IL-2 receptor antagonist. A benefit, in terms of better renal function, was not apparent in the treatment arm with de novo use of sirolimus without cyclosporine. These findings were also observed in a similar treatment group of another clinical trial. | ||
=====Increased Risk of Calcineurin Inhibitor-Induced Hemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura/Thrombotic Microangiopathy (HUS/TTP/TMA)===== | =====Increased Risk of Calcineurin Inhibitor-Induced Hemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura/Thrombotic Microangiopathy (HUS/TTP/TMA)===== | ||
* The concomitant use of | * The concomitant use of sirolimus with a calcineurin inhibitor may increase the risk of calcineurin inhibitor-induced hemolytic uremic syndrome/thrombotic thrombocytopenic purpura/thrombotic microangiopathy (HUS/TTP/TMA). | ||
=====Antimicrobial Prophylaxis===== | =====Antimicrobial Prophylaxis===== | ||
* Cases of Pneumocystis carinii pneumonia have been reported in patients not receiving antimicrobial prophylaxis. Therefore, antimicrobial prophylaxis for Pneumocystis carinii pneumonia should be administered for 1 year following transplantation. | * Cases of Pneumocystis carinii pneumonia have been reported in patients not receiving antimicrobial prophylaxis. Therefore, antimicrobial prophylaxis for Pneumocystis carinii pneumonia should be administered for 1 year following transplantation. | ||
| Line 130: | Line 130: | ||
* Patients on immunosuppressive therapy are at increased risk for skin cancer. Exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor. | * Patients on immunosuppressive therapy are at increased risk for skin cancer. Exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor. | ||
=====Interaction with Strong Inhibitors and Inducers of CYP3A4 and/or P-gp===== | =====Interaction with Strong Inhibitors and Inducers of CYP3A4 and/or P-gp===== | ||
* Co-administration of | * Co-administration of sirolimus with strong inhibitors of CYP3A4 and/or P-gp (such as ketoconazole, voriconazole, itraconazole, erythromycin, telithromycin, or clarithromycin) or strong inducers of CYP3A4 and/or P-gp (such as rifampin or rifabutin) is not recommended | ||
|clinicalTrials=* The following adverse reactions are discussed in greater detail in other sections of the label. | |clinicalTrials=* The following adverse reactions are discussed in greater detail in other sections of the label. | ||
* Increased susceptibility to infection, lymphoma, and malignancy | * Increased susceptibility to infection, lymphoma, and malignancy | ||
| Line 140: | Line 140: | ||
* Fluid Accumulation and Wound Healing. | * Fluid Accumulation and Wound Healing. | ||
* Hypertriglyceridemia, hypercholesterolemia | * Hypertriglyceridemia, hypercholesterolemia | ||
* Decline in renal function in long-term combination of cyclosporine with | * Decline in renal function in long-term combination of cyclosporine with sirolimus | ||
* Proteinuria | * Proteinuria | ||
* Interstitial lung disease | * Interstitial lung disease | ||
* Increased risk of calcineurin inhibitor-induced hemolytic uremic syndrome/thrombotic thrombocytopenic purpura/thrombotic microangiopathy (HUS/TTP/TMA). | * Increased risk of calcineurin inhibitor-induced hemolytic uremic syndrome/thrombotic thrombocytopenic purpura/thrombotic microangiopathy (HUS/TTP/TMA). | ||
* The most common (≥ 30%) adverse reactions observed with | * The most common (≥ 30%) adverse reactions observed with sirolimus in clinical studies are: peripheral edema, hypertriglyceridemia, hypertension, hypercholesterolemia, creatinine increased, constipation, abdominal pain, diarrhea, headache, fever, urinary tract infection, anemia, nausea, arthralgia, pain, and thrombocytopenia. | ||
* The following adverse reactions resulted in a rate of discontinuation of > 5% in clinical trials: creatinine increased, hypertriglyceridemia, and thrombotic thrombocytopenic purpura (TTP). | * The following adverse reactions resulted in a rate of discontinuation of > 5% in clinical trials: creatinine increased, hypertriglyceridemia, and thrombotic thrombocytopenic purpura (TTP). | ||
=====Clinical Studies Experience in Prophylaxis of Organ Rejection Following Renal Transplantation===== | =====Clinical Studies Experience in Prophylaxis of Organ Rejection Following Renal Transplantation===== | ||
* The safety and efficacy of | * The safety and efficacy of sirolimus Oral Solution for the prevention of organ rejection following renal transplantation were assessed in two randomized, double-blind, multicenter, controlled trials. The safety profiles in the two studies were similar. | ||
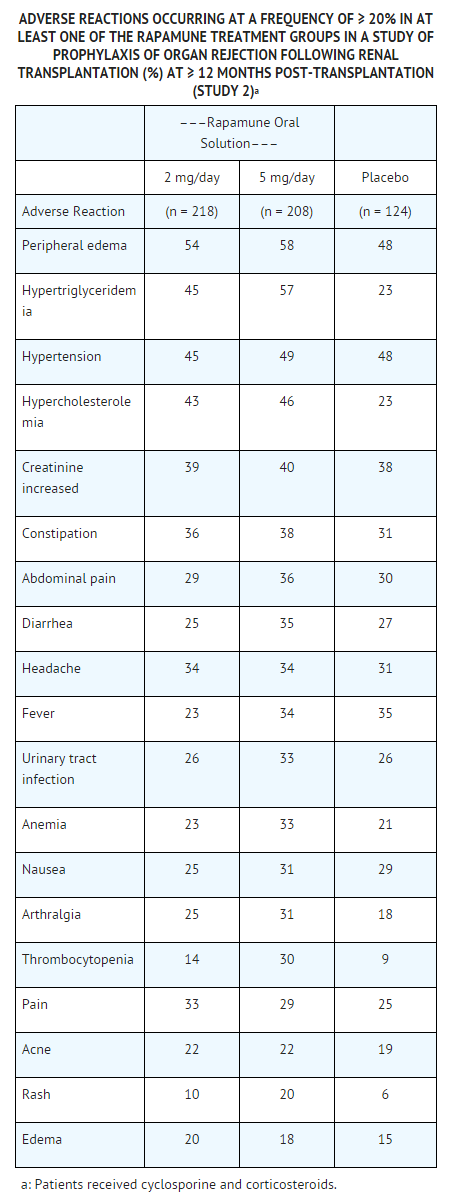
* The incidence of adverse reactions in the randomized, double-blind, multicenter, placebo-controlled trial (Study 2) in which 219 renal transplant patients received | * The incidence of adverse reactions in the randomized, double-blind, multicenter, placebo-controlled trial (Study 2) in which 219 renal transplant patients received sirolimus Oral Solution 2 mg/day, 208 received sirolimus Oral Solution 5 mg/day, and 124 received placebo is presented in the TABLE below. The study population had a mean age of 46 years (range 15 to 71 years), the distribution was 67% male, and the composition by race was: White (78%), Black (11%), Asian (3%), Hispanic (2%), and Other (5%). All patients were treated with cyclosporine and corticosteroids. Data (≥ 12 months post-transplant) presented in the following TABLE show the adverse reactions that occurred in at least one of the sirolimus treatment groups with an incidence of ≥ 20%. | ||
* The safety profile of the tablet did not differ from that of the oral solution formulation. | * The safety profile of the tablet did not differ from that of the oral solution formulation. | ||
* In general, adverse reactions related to the administration of | * In general, adverse reactions related to the administration of sirolimus were dependent on dose/concentration. Although a daily maintenance dose of 5 mg, with a loading dose of 15 mg, was shown to be safe and effective, no efficacy advantage over the 2 mg dose could be established for renal transplant patients. | ||
* Patients receiving 2 mg of | * Patients receiving 2 mg of sirolimus Oral Solution per day demonstrated an overall better safety profile than did patients receiving 5 mg of sirolimus Oral Solution per day. | ||
* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice. | * Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice. | ||
: [[File:Sirolimus 01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 172: | Line 172: | ||
* Melanoma, squamous cell carcinoma, basal cell carcinoma. | * Melanoma, squamous cell carcinoma, basal cell carcinoma. | ||
=====Urogenital System===== | =====Urogenital System===== | ||
* Pyelonephritis, decline in renal function (creatinine increased) in long-term combination of cyclosporine with | * Pyelonephritis, decline in renal function (creatinine increased) in long-term combination of cyclosporine with sirolimus. | ||
* Less frequently (< 3%) occurring adverse reactions included: lymphoma/post-transplant lymphoproliferative disorder, mycobacterial infections (including M. tuberculosis), pancreatitis, cytomegalovirus (CMV), and Epstein-Barr virus. | * Less frequently (< 3%) occurring adverse reactions included: lymphoma/post-transplant lymphoproliferative disorder, mycobacterial infections (including M. tuberculosis), pancreatitis, cytomegalovirus (CMV), and Epstein-Barr virus. | ||
=====Increased Serum Cholesterol and Triglycerides===== | =====Increased Serum Cholesterol and Triglycerides===== | ||
* The use of | * The use of sirolimus in renal transplant patients was associated with increased serum cholesterol and triglycerides that may require treatment. | ||
* In Studies 1 and 2, in de novo renal transplant patients who began the study with fasting, total serum cholesterol< 200 mg/dL or fasting, total serum triglycerides < 200 mg/dL, there was an increased incidence of hypercholesterolemia (fasting serum cholesterol > 240 mg/dL) or hypertriglyceridemia (fasting serum triglycerides > 500 mg/dL), respectively, in patients receiving both | * In Studies 1 and 2, in de novo renal transplant patients who began the study with fasting, total serum cholesterol< 200 mg/dL or fasting, total serum triglycerides < 200 mg/dL, there was an increased incidence of hypercholesterolemia (fasting serum cholesterol > 240 mg/dL) or hypertriglyceridemia (fasting serum triglycerides > 500 mg/dL), respectively, in patients receiving both sirolimus 2 mg and sirolimus 5 mg compared with azathioprine and placebo controls. | ||
* Treatment of new-onset hypercholesterolemia with lipid-lowering agents was required in 42‑52% of patients enrolled in the | * Treatment of new-onset hypercholesterolemia with lipid-lowering agents was required in 42‑52% of patients enrolled in the sirolimus arms of Studies 1 and 2 compared with 16% of patients in the placebo arm and 22% of patients in the azathioprine arm. | ||
=====Abnormal Healing===== | =====Abnormal Healing===== | ||
* Abnormal healing events following transplant surgery include fascial dehiscence, incisional hernia, and anastomosis disruption (e.g., wound, vascular, airway, ureteral, biliary). | * Abnormal healing events following transplant surgery include fascial dehiscence, incisional hernia, and anastomosis disruption (e.g., wound, vascular, airway, ureteral, biliary). | ||
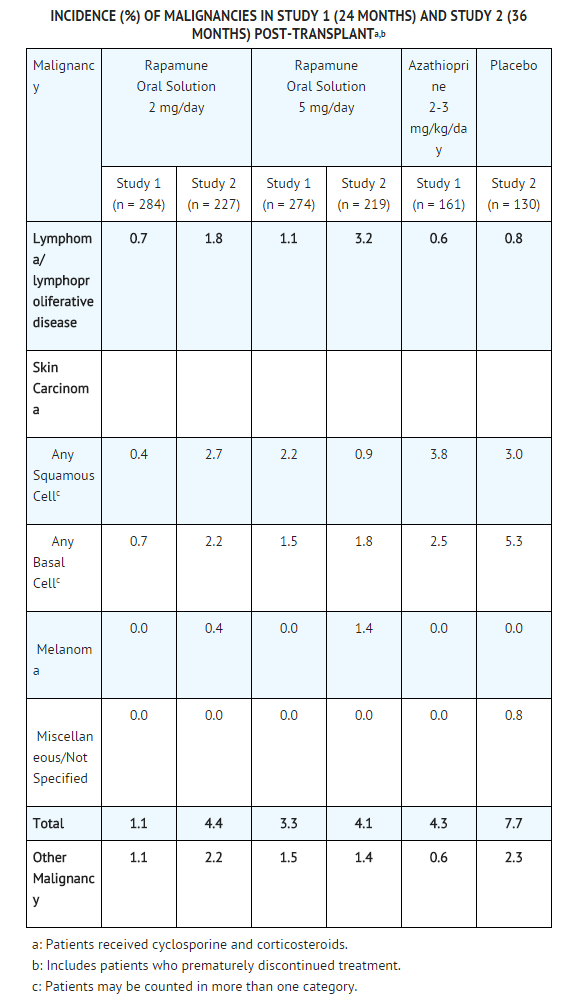
| Line 184: | Line 184: | ||
* At 24 months (Study 1) and 36 months (Study 2), there were no significant differences among treatment groups. | * At 24 months (Study 1) and 36 months (Study 2), there were no significant differences among treatment groups. | ||
: [[File:Sirolimus 02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
===== | =====Sirolimus Following Cyclosporine Withdrawal===== | ||
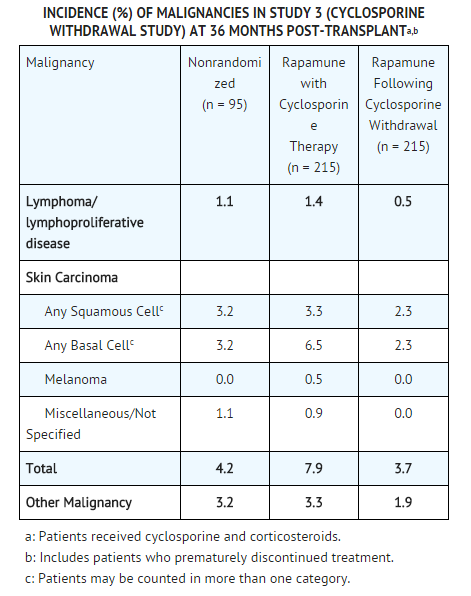
* The incidence of adverse reactions was determined through 36 months in a randomized, multicenter, controlled trial (Study 3) in which 215 renal transplant patients received | * The incidence of adverse reactions was determined through 36 months in a randomized, multicenter, controlled trial (Study 3) in which 215 renal transplant patients received sirolimus as a maintenance regimen following cyclosporine withdrawal, and 215 patients received sirolimus with cyclosporine therapy. | ||
* All patients were treated with corticosteroids. The safety profile prior to randomization (start of cyclosporine withdrawal) was similar to that of the 2 mg | * All patients were treated with corticosteroids. The safety profile prior to randomization (start of cyclosporine withdrawal) was similar to that of the 2 mg sirolimus groups in Studies 1 and 2. | ||
* Following randomization (at 3 months), patients who had cyclosporine eliminated from their therapy experienced higher incidences of the following adverse reactions: abnormal liver function tests (including increased AST/SGOT and increased ALT/SGPT), hypokalemia, thrombocytopenia, and abnormal healing. Conversely, the incidence of the following adverse events was higher in patients who remained on cyclosporine than those who had cyclosporine withdrawn from therapy: hypertension, cyclosporine toxicity, increased creatinine, abnormal kidney function, toxic nephropathy, edema, hyperkalemia, hyperuricemia, and gum hyperplasia. Mean systolic and diastolic blood pressure improved significantly following cyclosporine withdrawal. | * Following randomization (at 3 months), patients who had cyclosporine eliminated from their therapy experienced higher incidences of the following adverse reactions: abnormal liver function tests (including increased AST/SGOT and increased ALT/SGPT), hypokalemia, thrombocytopenia, and abnormal healing. Conversely, the incidence of the following adverse events was higher in patients who remained on cyclosporine than those who had cyclosporine withdrawn from therapy: hypertension, cyclosporine toxicity, increased creatinine, abnormal kidney function, toxic nephropathy, edema, hyperkalemia, hyperuricemia, and gum hyperplasia. Mean systolic and diastolic blood pressure improved significantly following cyclosporine withdrawal. | ||
=====Malignancies===== | =====Malignancies===== | ||
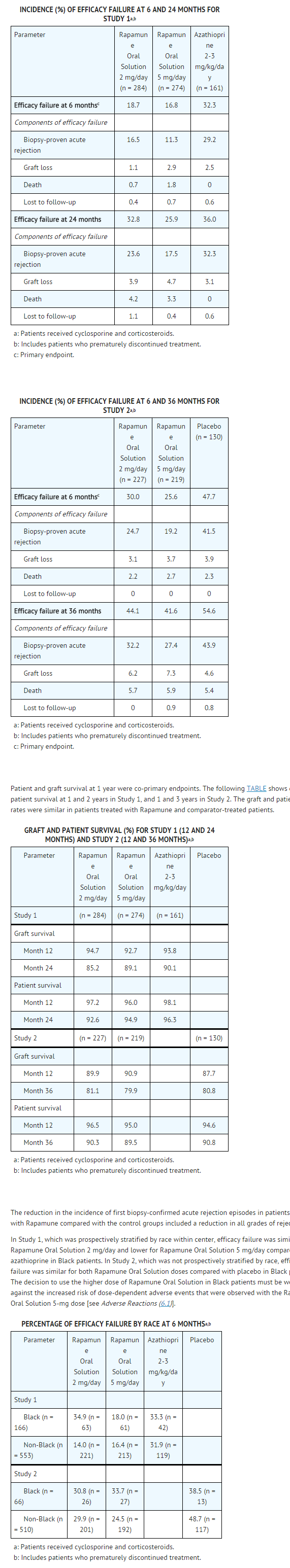
* The incidence of malignancies in Study 3 [see Clinical Studies (14.2)] is presented in the TABLE following. | * The incidence of malignancies in Study 3 [see Clinical Studies (14.2)] is presented in the TABLE following. | ||
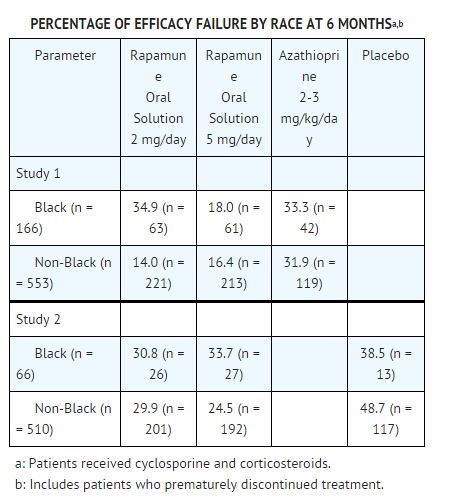
* In Study 3, the incidence of lymphoma/lymphoproliferative disease was similar in all treatment groups. The overall incidence of malignancy was higher in patients receiving | * In Study 3, the incidence of lymphoma/lymphoproliferative disease was similar in all treatment groups. The overall incidence of malignancy was higher in patients receiving sirolimus plus cyclosporine compared with patients who had cyclosporine withdrawn. Conclusions regarding these differences in the incidence of malignancy could not be made because Study 3 was not designed to consider malignancy risk factors or systematically screen subjects for malignancy. In addition, more patients in the sirolimus with cyclosporine group had a pretransplantation history of skin carcinoma. | ||
: [[File:Sirolimus 03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
=====High-Immunologic Risk Patients===== | =====High-Immunologic Risk Patients===== | ||
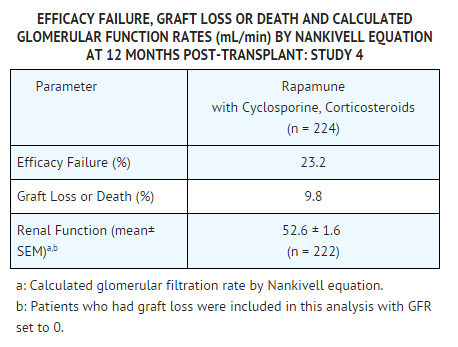
* Safety was assessed in 224 patients who received at least one dose of sirolimus with cyclosporine [see Clinical Studies (14.3)]. Overall, the incidence and nature of adverse events was similar to those seen in previous combination studies with | * Safety was assessed in 224 patients who received at least one dose of sirolimus with cyclosporine [see Clinical Studies (14.3)]. Overall, the incidence and nature of adverse events was similar to those seen in previous combination studies with sirolimus. The incidence of malignancy was 1.3% at 12 months. | ||
=====Conversion from Calcineurin Inhibitors to | =====Conversion from Calcineurin Inhibitors to sirolimus in Maintenance Renal Transplant Population===== | ||
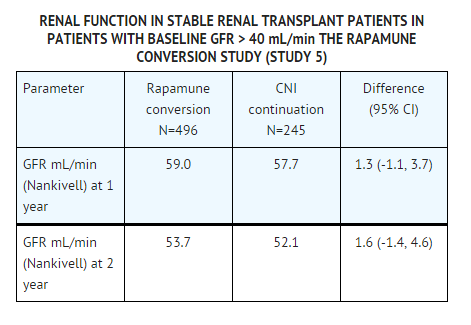
* The safety and efficacy of conversion from calcineurin inhibitors to | * The safety and efficacy of conversion from calcineurin inhibitors to sirolimus in maintenance renal transplant population have not been established [see Clinical Studies (14.4)]. In an ongoing study evaluating the safety and efficacy of conversion from calcineurin inhibitors to sirolimus (initial target sirolimus concentrations of 12-20 ng/mL, and then 8-20 ng/mL, by chromatographic assay) in maintenance renal transplant patients, enrollment was stopped in the subset of patients (n = 87) with a baseline glomerular filtration rate of less than 40 mL/min. There was a higher rate of serious adverse events, including pneumonia, acute rejection, graft loss and death, in this stratum of the sirolimus treatment arm. | ||
* The subset of patients with a baseline glomerular filtration rate of less than 40 mL/min had 2 years of follow-up after randomization. In this population, the rate of pneumonia was 15/58 vs. 4/29, graft loss (excluding death with functioning graft loss) was 13/58 vs. 9/29, and death was 9/58 vs. 1/29 in the sirolimus conversion group and CNI continuation group, respectively. | * The subset of patients with a baseline glomerular filtration rate of less than 40 mL/min had 2 years of follow-up after randomization. In this population, the rate of pneumonia was 15/58 vs. 4/29, graft loss (excluding death with functioning graft loss) was 13/58 vs. 9/29, and death was 9/58 vs. 1/29 in the sirolimus conversion group and CNI continuation group, respectively. | ||
* In the subset of patients with a baseline glomerular filtration rate of greater than 40 mL/min, there was no benefit associated with conversion with regard to improvement in renal function and a greater incidence of proteinuria in the | * In the subset of patients with a baseline glomerular filtration rate of greater than 40 mL/min, there was no benefit associated with conversion with regard to improvement in renal function and a greater incidence of proteinuria in the sirolimus conversion arm. | ||
* Overall in this study, a 5-fold increase in the reports of tuberculosis among sirolimus (11/551) and comparator (1/273) treatment groups was observed with 2:1 randomization scheme. | * Overall in this study, a 5-fold increase in the reports of tuberculosis among sirolimus (11/551) and comparator (1/273) treatment groups was observed with 2:1 randomization scheme. | ||
=====Pediatrics===== | =====Pediatrics===== | ||
* Safety was assessed in a controlled clinical trial in pediatric (< 18 years of age) renal transplant patients considered at high-immunologic risk, defined as a history of one or more acute allograft rejection episodes and/or the presence of chronic allograft nephropathy on a renal biopsy. | * Safety was assessed in a controlled clinical trial in pediatric (< 18 years of age) renal transplant patients considered at high-immunologic risk, defined as a history of one or more acute allograft rejection episodes and/or the presence of chronic allograft nephropathy on a renal biopsy. | ||
* The use of | * The use of sirolimus in combination with calcineurin inhibitors and corticosteroids was associated with a higher incidence of deterioration of renal function (creatinine increased) compared to calcineurin inhibitor-based therapy, serum lipid abnormalities (including, but not limited to, increased serum triglycerides and cholesterol), and urinary tract infections. | ||
|postmarketing=* The following adverse reactions have been identified during post-approval use of | |postmarketing=* The following adverse reactions have been identified during post-approval use of sirolimus. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
=====Body as a Whole – Lymphedema===== | =====Body as a Whole – Lymphedema===== | ||
* Cardiovascular – Pericardial effusion (including hemodynamically significant effusions and tamponade requiring intervention in children and adults). | * Cardiovascular – Pericardial effusion (including hemodynamically significant effusions and tamponade requiring intervention in children and adults). | ||
* Hematological/Lymphatic – The concomitant use of | * Hematological/Lymphatic – The concomitant use of sirolimus with a calcineurin inhibitor may increase the risk of calcineurin inhibitor-induced HUS/TTP/TMA; pancytopenia, neutropenia. | ||
=====Hepatobiliary Disorders===== | =====Hepatobiliary Disorders===== | ||
* Hepatotoxicity, including fatal hepatic necrosis, with elevated sirolimus trough concentrations. | * Hepatotoxicity, including fatal hepatic necrosis, with elevated sirolimus trough concentrations. | ||
| Line 215: | Line 215: | ||
* Liver function test abnormal, AST/SGOT increased, ALT/SGPT increased, hypophosphatemia, hyperglycemia. | * Liver function test abnormal, AST/SGOT increased, ALT/SGPT increased, hypophosphatemia, hyperglycemia. | ||
=====Respiratory===== | =====Respiratory===== | ||
* Cases of interstitial lung disease (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving immunosuppressive regimens including | * Cases of interstitial lung disease (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving immunosuppressive regimens including sirolimus. In some cases, the interstitial lung disease has resolved upon discontinuation or dose reduction of sirolimus. The risk may be increased as the sirolimus trough concentration increases; pulmonary hemorrhage; pleural effusion; alveolar proteinosis | ||
=====Skin===== | =====Skin===== | ||
* Exfoliative dermatitis. | * Exfoliative dermatitis. | ||
=====Urogenital===== | =====Urogenital===== | ||
* Nephrotic syndrome, proteinuria, focal segmental glomerulosclerosis. Azoospermia has been reported with the use of | * Nephrotic syndrome, proteinuria, focal segmental glomerulosclerosis. Azoospermia has been reported with the use of sirolimus and has been reversible upon discontinuation of sirolimus in most cases. | ||
|drugInteractions=* Sirolimus is known to be a substrate for both cytochrome P‑450 3A4 (CYP3A4) and p‑glycoprotein (P‑gp). Inducers of CYP3A4 and P‑gp may decrease sirolimus concentrations whereas inhibitors of CYP3A4 and P‑gp may increase sirolimus concentrations. | |drugInteractions=* Sirolimus is known to be a substrate for both cytochrome P‑450 3A4 (CYP3A4) and p‑glycoprotein (P‑gp). Inducers of CYP3A4 and P‑gp may decrease sirolimus concentrations whereas inhibitors of CYP3A4 and P‑gp may increase sirolimus concentrations. | ||
=====Use with Cyclosporine===== | =====Use with Cyclosporine===== | ||
* Cyclosporine, a substrate and inhibitor of CYP3A4 and P‑gp, was demonstrated to increase sirolimus concentrations when co‑administered with sirolimus. In order to diminish the effect of this interaction with cyclosporine, it is recommended that | * Cyclosporine, a substrate and inhibitor of CYP3A4 and P‑gp, was demonstrated to increase sirolimus concentrations when co‑administered with sirolimus. In order to diminish the effect of this interaction with cyclosporine, it is recommended that sirolimus be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and/or cyclosporine capsules (MODIFIED). | ||
* If cyclosporine is withdrawn from combination therapy with | * If cyclosporine is withdrawn from combination therapy with sirolimus, higher doses of sirolimus are needed to maintain the recommended sirolimus trough concentration ranges. | ||
* Strong Inducers and Strong Inhibitors of CYP3A4 and P-gp | * Strong Inducers and Strong Inhibitors of CYP3A4 and P-gp | ||
Avoid concomitant use of sirolimus with strong inducers (e.g., rifampin, rifabutin) and strong inhibitors (e.g., ketoconazole, voriconazole, itraconazole, erythromycin, telithromycin, clarithromycin) of CYP3A4 and P-gp. Alternative agents with lesser interaction potential with sirolimus should be considered. | Avoid concomitant use of sirolimus with strong inducers (e.g., rifampin, rifabutin) and strong inhibitors (e.g., ketoconazole, voriconazole, itraconazole, erythromycin, telithromycin, clarithromycin) of CYP3A4 and P-gp. Alternative agents with lesser interaction potential with sirolimus should be considered. | ||
=====Grapefruit Juice===== | =====Grapefruit Juice===== | ||
* Because grapefruit juice inhibits the CYP3A4-mediated metabolism of sirolimus, it must not be taken with or be used for dilution of | * Because grapefruit juice inhibits the CYP3A4-mediated metabolism of sirolimus, it must not be taken with or be used for dilution of sirolimus. | ||
=====Inducers or Inhibitors of CYP3A4 and P-gp===== | =====Inducers or Inhibitors of CYP3A4 and P-gp===== | ||
* Exercise caution when using sirolimus with drugs or agents that are modulators of CYP3A4 and P-gp. The dosage of | * Exercise caution when using sirolimus with drugs or agents that are modulators of CYP3A4 and P-gp. The dosage of sirolimus and/or the co-administered drug may need to be adjusted. | ||
* Drugs that could increase sirolimus blood concentrations: | * Drugs that could increase sirolimus blood concentrations: | ||
Bromocriptione, cimetidine, cisapride, clotrimazole, danazol, diltiazem, fluconazole, HIV‑protease inhibitors (e.g., ritonavir, indinavir), metoclopramide, nicardipine, troleandomycin, verapamil | Bromocriptione, cimetidine, cisapride, clotrimazole, danazol, diltiazem, fluconazole, HIV‑protease inhibitors (e.g., ritonavir, indinavir), metoclopramide, nicardipine, troleandomycin, verapamil | ||
Drugs and other agents that could decrease sirolimus concentrations: | Drugs and other agents that could decrease sirolimus concentrations: | ||
* Carbamazepine, phenobarbital, phenytoin, rifapentine, St. John's Wort (Hypericum perforatum) | * Carbamazepine, phenobarbital, phenytoin, rifapentine, St. John's Wort (Hypericum perforatum) | ||
* Drugs with concentrations that could increase when given with | * Drugs with concentrations that could increase when given with sirolimus: | ||
:* Verapamil | :* Verapamil | ||
=====Vaccination===== | =====Vaccination===== | ||
* Immunosuppressants may affect response to vaccination. Therefore, during treatment with | * Immunosuppressants may affect response to vaccination. Therefore, during treatment with sirolimus, vaccination may be less effective. The use of live vaccines should be avoided; live vaccines may include, but are not limited to, the following: measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid. | ||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=* Sirolimus was embryo/fetotoxic in rats when given in doses approximately 0.2 to 0.5 the human doses (adjusted for body surface area). Embryo/fetotoxicity was manifested as mortality and reduced fetal weights (with associated delays in skeletal ossification). However, no teratogenesis was evident. In combination with cyclosporine, rats had increased embryo/feto mortality compared with sirolimus alone. There were no effects on rabbit development at a maternally toxic dosage approximately 0.3 to 0.8 times the human doses (adjusted for body surface area). There are no adequate and well‑controlled studies in pregnant women. Effective contraception must be initiated before | |useInPregnancyFDA=* Sirolimus was embryo/fetotoxic in rats when given in doses approximately 0.2 to 0.5 the human doses (adjusted for body surface area). Embryo/fetotoxicity was manifested as mortality and reduced fetal weights (with associated delays in skeletal ossification). However, no teratogenesis was evident. In combination with cyclosporine, rats had increased embryo/feto mortality compared with sirolimus alone. There were no effects on rabbit development at a maternally toxic dosage approximately 0.3 to 0.8 times the human doses (adjusted for body surface area). There are no adequate and well‑controlled studies in pregnant women. Effective contraception must be initiated before sirolimus therapy, during sirolimus therapy, and for 12 weeks after sirolimus therapy has been stopped. Sirolimus should be used during pregnancy only if the potential benefit outweighs the potential risk to the embryo/fetus. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
| Line 245: | Line 245: | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=* Sirolimus is excreted in trace amounts in milk of lactating rats. It is not known whether sirolimus is excreted in human milk. The pharmacokinetic and safety profiles of sirolimus in infants are not known. Because many drugs are excreted in human milk, and because of the potential for adverse reactions in nursing infants from sirolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |useInNursing=* Sirolimus is excreted in trace amounts in milk of lactating rats. It is not known whether sirolimus is excreted in human milk. The pharmacokinetic and safety profiles of sirolimus in infants are not known. Because many drugs are excreted in human milk, and because of the potential for adverse reactions in nursing infants from sirolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | ||
|useInPed=* The safety and efficacy of | |useInPed=* The safety and efficacy of sirolimus in pediatric patients < 13 years have not been established. | ||
* The safety and efficacy of | * The safety and efficacy of sirolimus Oral Solution and sirolimus Tablets have been established in children ≥ 13 years judged to be at low- to moderate-immunologic risk. Use of sirolimus Oral Solution and sirolimus Tablets in this subpopulation of children ≥ 13 years is supported by evidence from adequate and well-controlled trials of sirolimus Oral Solution in adults with additional pharmacokinetic data in pediatric renal transplantation patients. | ||
* Safety and efficacy information from a controlled clinical trial in pediatric and adolescent (< 18 years of age) renal transplant patients judged to be at high-immunologic risk, defined as a history of one or more acute rejection episodes and/or the presence of chronic allograft nephropathy, do not support the chronic use of | * Safety and efficacy information from a controlled clinical trial in pediatric and adolescent (< 18 years of age) renal transplant patients judged to be at high-immunologic risk, defined as a history of one or more acute rejection episodes and/or the presence of chronic allograft nephropathy, do not support the chronic use of sirolimus Oral Solution or Tablets in combination with calcineurin inhibitors and corticosteroids, due to the higher incidence of lipid abnormalities and deterioration of renal function associated with these immunosuppressive regimens compared to calcineurin inhibitors, without increased benefit with respect to acute rejection, graft survival, or patient survival. | ||
|useInGeri=* Clinical studies of | |useInGeri=* Clinical studies of sirolimus Oral Solution or Tablets did not include sufficient numbers of patients ≥ 65 years to determine whether they respond differently from younger patients. Data pertaining to sirolimus trough concentrations suggest that dose adjustments based upon age in geriatric renal patients are not necessary. Differences in responses between the elderly and younger patients have not been identified. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, or cardiac function, and of concomitant disease or other drug therapy. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair=* Dosage adjustment is not required in patients with renal impairment . | |useInRenalImpair=* Dosage adjustment is not required in patients with renal impairment . | ||
|useInHepaticImpair=* The maintenance dose of | |useInHepaticImpair=* The maintenance dose of sirolimus should be reduced in patients with hepatic impairment. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
| Line 258: | Line 258: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* Oral | ||
|monitoring=* Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the | |monitoring=* Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the sirolimus dosage form is made, and during concurrent administration of strong CYP3A4 inducers and inhibitors. | ||
* Therapeutic drug monitoring should not be the sole basis for adjusting | * Therapeutic drug monitoring should not be the sole basis for adjusting sirolimus therapy. Careful attention should be made to clinical signs/symptoms, tissue biopsy findings, and laboratory parameters. | ||
* When used in combination with cyclosporine, sirolimus trough concentrations should be maintained within the target-range. | * When used in combination with cyclosporine, sirolimus trough concentrations should be maintained within the target-range. | ||
* Following cyclosporine withdrawal in transplant patients at low- to moderate-immunologic risk, the target sirolimus trough concentrations should be 16 to 24 ng/mL for the first year following transplantation. Thereafter, the target sirolimus concentrations should be 12 to 20 ng/mL. | * Following cyclosporine withdrawal in transplant patients at low- to moderate-immunologic risk, the target sirolimus trough concentrations should be 16 to 24 ng/mL for the first year following transplantation. Thereafter, the target sirolimus concentrations should be 12 to 20 ng/mL. | ||
| Line 268: | Line 268: | ||
* Renal function should be monitored, and appropriate adjustment of the immunosuppressive regimen should be considered in patients with elevated or increasing serum creatinine levels. | * Renal function should be monitored, and appropriate adjustment of the immunosuppressive regimen should be considered in patients with elevated or increasing serum creatinine levels. | ||
* Periodic quantitative monitoring of urinary protein excretion is recommended. | * Periodic quantitative monitoring of urinary protein excretion is recommended. | ||
* During | * During sirolimus therapy with cyclosporine, patients administered an HMG-CoA reductase inhibitor and/or fibrate should be monitored for the possible development of rhabdomyolysis and other adverse effects, as described in the respective labeling for these agents. | ||
* Any patient who is administered | * Any patient who is administered sirolimus should be monitored for hyperlipidemia. | ||
* Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the | * Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the sirolimus dosage form is made, and during concurrent administration of strong CYP3A4 inducers and inhibitors. | ||
* Once | * Once sirolimus maintenance dose is adjusted, patients should continue on the new maintenance dose for at least 7 to 14 days before further dosage adjustment with concentration monitoring. | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose=* Reports of overdose with | |overdose=* Reports of overdose with sirolimus have been received; however, experience has been limited. In general, the adverse effects of overdose are consistent with those listed in the adverse reactions section. | ||
* General supportive measures should be followed in all cases of overdose. Based on the low aqueous solubility and high erythrocyte and plasma protein binding of sirolimus, it is anticipated that sirolimus is not dialyzable to any significant extent. In mice and rats, the acute oral LD50 was greater than 800 mg/kg. | * General supportive measures should be followed in all cases of overdose. Based on the low aqueous solubility and high erythrocyte and plasma protein binding of sirolimus, it is anticipated that sirolimus is not dialyzable to any significant extent. In mice and rats, the acute oral LD50 was greater than 800 mg/kg. | ||
|drugBox={{drugbox2 | |drugBox={{drugbox2 | ||
| Line 318: | Line 318: | ||
| metabolism = Hepatic | | metabolism = Hepatic | ||
| elimination_half-life = 57–63 hours | | elimination_half-life = 57–63 hours | ||
| licence_EU = | | licence_EU = sirolimus | ||
| licence_US = Sirolimus | | licence_US = Sirolimus | ||
| pregnancy_AU = C | | pregnancy_AU = C | ||
| Line 329: | Line 329: | ||
* Studies in experimental models show that sirolimus prolongs allograft (kidney, heart, skin, islet, small bowel, pancreatico-duodenal, and bone marrow) survival in mice, rats, pigs, and/or primates. Sirolimus reverses acute rejection of heart and kidney allografts in rats and prolongs the graft survival in presensitized rats. In some studies, the immunosuppressive effect of sirolimus lasts up to 6 months after discontinuation of therapy. This tolerization effect is alloantigen-specific. | * Studies in experimental models show that sirolimus prolongs allograft (kidney, heart, skin, islet, small bowel, pancreatico-duodenal, and bone marrow) survival in mice, rats, pigs, and/or primates. Sirolimus reverses acute rejection of heart and kidney allografts in rats and prolongs the graft survival in presensitized rats. In some studies, the immunosuppressive effect of sirolimus lasts up to 6 months after discontinuation of therapy. This tolerization effect is alloantigen-specific. | ||
* In rodent models of autoimmune disease, sirolimus suppresses immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis. | * In rodent models of autoimmune disease, sirolimus suppresses immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis. | ||
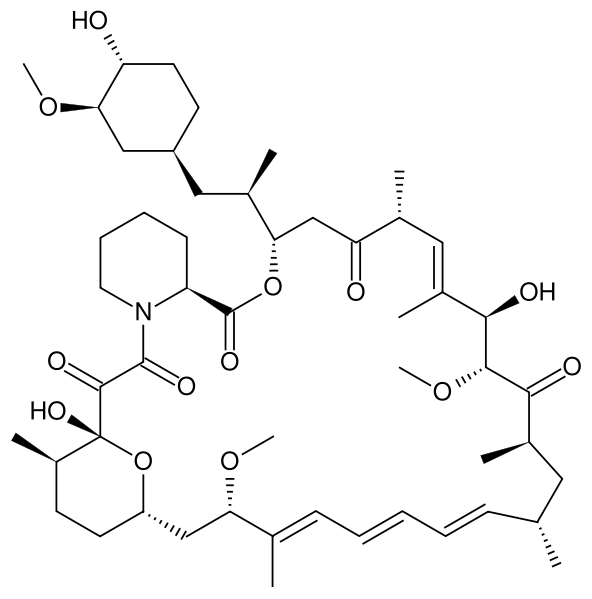
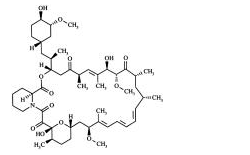
|structure=* | |structure=* Sirolimus is an immunosuppressive agent. Sirolimus is a macrocyclic lactone produced by Streptomyces hygroscopicus. The chemical name of sirolimus (also known as rapamycin) is (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34, 34a-hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4] oxaazacyclohentriacontine-1,5,11,28,29 (4H,6H,31H)-pentone. Its molecular formula is C51H79NO13 and its molecular weight is 914.2. The structural formula of sirolimus is illustrated as follows. | ||
: [[File:Sirolimus 17.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 17.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Sirolimus is a white to off-white powder and is insoluble in water, but freely soluble in benzyl alcohol, chloroform, acetone, and acetonitrile. | * Sirolimus is a white to off-white powder and is insoluble in water, but freely soluble in benzyl alcohol, chloroform, acetone, and acetonitrile. | ||
* | * Sirolimus is available for administration as an oral solution containing 1 mg/mL sirolimus. Sirolimus is also available as a white, triangular-shaped tablet containing 1 mg sirolimus, and as a yellow-to-beige triangular-shaped tablet containing 2 mg sirolimus. | ||
* The inactive ingredients in | * The inactive ingredients in sirolimus Oral Solution are Phosal 50 PG® (phosphatidylcholine, propylene glycol, mono- and di-glycerides, ethanol, soy fatty acids, and ascorbyl palmitate) and polysorbate 80. Sirolimus Oral Solution contains 1.5% - 2.5% ethanol. | ||
* The inactive ingredients in | * The inactive ingredients in sirolimus Tablets include sucrose, lactose, polyethylene glycol 8000, calcium sulfate, microcrystalline cellulose, pharmaceutical glaze, talc, titanium dioxide, magnesium stearate, povidone, poloxamer 188, polyethylene glycol 20,000, glyceryl monooleate, carnauba wax, dl-alpha tocopherol, and other ingredients. The 2 mg dosage strength also contains iron oxide yellow 10 and iron oxide brown 70. | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=* Orally-administered | |PD=* Orally-administered sirolimus, at doses of 2 mg/day and 5 mg/day, significantly reduced the incidence of organ rejection in low- to moderate-immunologic risk renal transplant patients at 6 months following transplantation compared with either azathioprine or placebo. There was no demonstrable efficacy advantage of a daily maintenance dose of 5 mg with a loading dose of 15 mg over a daily maintenance dose of 2 mg with a loading dose of 6 mg. Therapeutic drug monitoring should be used to maintain sirolimus drug levels within the target-range. | ||
|PK=* Sirolimus pharmacokinetics activity have been determined following oral administration in healthy subjects, pediatric patients, hepatically impaired patients, and renal transplant patients. | |PK=* Sirolimus pharmacokinetics activity have been determined following oral administration in healthy subjects, pediatric patients, hepatically impaired patients, and renal transplant patients. | ||
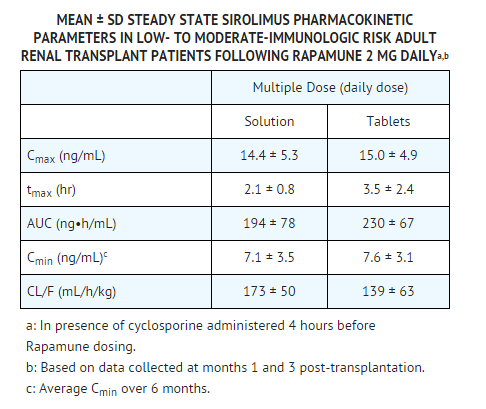
* The pharmacokinetic parameters of sirolimus in low- to moderate-immunologic risk adult renal transplant patients following multiple dosing with | * The pharmacokinetic parameters of sirolimus in low- to moderate-immunologic risk adult renal transplant patients following multiple dosing with sirolimus 2 mg daily, in combination with cyclosporine and corticosteroids, is summarized in the following TABLE. | ||
: [[File:Sirolimus 04.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 04.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Whole blood trough sirolimus concentrations, as measured by LC/MS/MS in renal transplant patients, were significantly correlated with AUCτ,ss. Upon repeated, twice-daily administration without an initial loading dose in a multiple-dose study, the average trough concentration of sirolimus increases approximately 2- to 3-fold over the initial 6 days of therapy, at which time steady-state is reached. A loading dose of 3 times the maintenance dose will provide near steady-state concentrations within 1 day in most patients. | * Whole blood trough sirolimus concentrations, as measured by LC/MS/MS in renal transplant patients, were significantly correlated with AUCτ,ss. Upon repeated, twice-daily administration without an initial loading dose in a multiple-dose study, the average trough concentration of sirolimus increases approximately 2- to 3-fold over the initial 6 days of therapy, at which time steady-state is reached. A loading dose of 3 times the maintenance dose will provide near steady-state concentrations within 1 day in most patients. | ||
=====Absorption===== | =====Absorption===== | ||
* Following administration of | * Following administration of sirolimus Oral Solution, the mean times to peak concentration (tmax) of sirolimus are approximately 1 hour and 2 hours in healthy subjects and renal transplant patients, respectively. The systemic availability of sirolimus is low, and was estimated to be approximately 14% after the administration of sirolimus Oral Solution. In healthy subjects, the mean bioavailability of sirolimus after administration of the tablet is approximately 27% higher relative to the solution. | ||
* Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of | * Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of sirolimus Oral Solution to stable renal transplant patients, are dose-proportional between 3 and 12 mg/m2. | ||
=====Food Effects===== | =====Food Effects===== | ||
* To minimize variability in sirolimus concentrations, both | * To minimize variability in sirolimus concentrations, both sirolimus Oral Solution and Tablets should be taken consistently with or without food. In healthy subjects, a high-fat meal (861.8 kcal, 54.9% kcal from fat) increased the mean total exposure (AUC) of sirolimus by 23 to 35%, compared with fasting. The effect of food on the mean sirolimus Cmax was inconsistent depending on the sirolimus dosage form evaluated. | ||
=====Distribution===== | =====Distribution===== | ||
* The mean (± SD) blood-to-plasma ratio of sirolimus was 36 ± 18 in stable renal allograft patients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution (Vss/F) of sirolimus is 12 ± 8 L/kg. Sirolimus is extensively bound (approximately 92%) to human plasma proteins, mainly serum albumin (97%), α1-acid glycoprotein, and lipoproteins. | * The mean (± SD) blood-to-plasma ratio of sirolimus was 36 ± 18 in stable renal allograft patients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution (Vss/F) of sirolimus is 12 ± 8 L/kg. Sirolimus is extensively bound (approximately 92%) to human plasma proteins, mainly serum albumin (97%), α1-acid glycoprotein, and lipoproteins. | ||
| Line 357: | Line 357: | ||
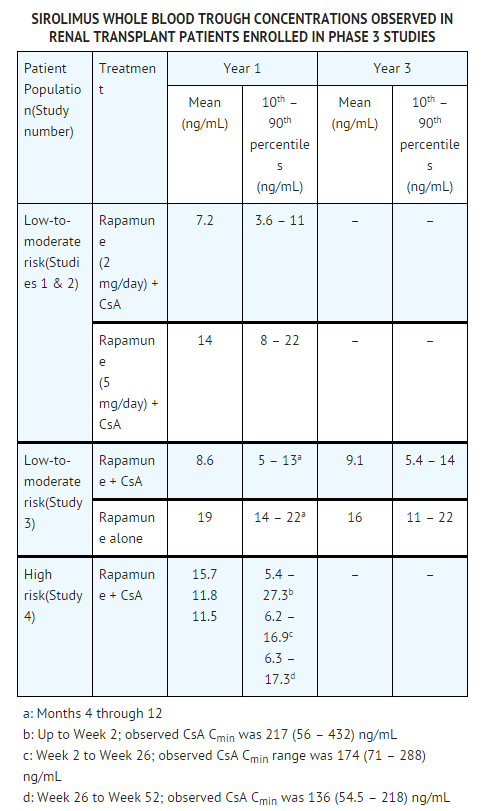
* The FOLLOWING sirolimus concentrations (chromatographic equivalent) were observed in phase 3 clinical studies. | * The FOLLOWING sirolimus concentrations (chromatographic equivalent) were observed in phase 3 clinical studies. | ||
: [[File:Sirolimus 05.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 05.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* The withdrawal of cyclosporine and concurrent increases in sirolimus trough concentrations to steady-state required approximately 6 weeks. Following cyclosporine withdrawal, larger | * The withdrawal of cyclosporine and concurrent increases in sirolimus trough concentrations to steady-state required approximately 6 weeks. Following cyclosporine withdrawal, larger sirolimus doses were required due to the absence of the inhibition of sirolimus metabolism and transport by cyclosporine and to achieve higher target sirolimus trough concentrations during concentration-controlled administration. | ||
====Pharmacokinetics in Specific Populations==== | ====Pharmacokinetics in Specific Populations==== | ||
=====Hepatic Impairment====== | =====Hepatic Impairment====== | ||
* | * Sirolimus was administered as a single, oral dose to subjects with normal hepatic function and to patients with Child-Pugh classification A (mild), B (moderate), or C (severe) hepatic impairment. Compared with the values in the normal hepatic function group, the patients with mild, moderate, and severe hepatic impairment had 43%, 94%, and 189% higher mean values for sirolimus AUC, respectively, with no statistically significant differences in mean Cmax. As the severity of hepatic impairment increased, there were steady increases in mean sirolimus t1/2, and decreases in the mean sirolimus clearance normalized for body weight (CL/F/kg). | ||
* The maintenance dose of | * The maintenance dose of sirolimus should be reduced by approximately one third in patients with mild‑to‑moderate hepatic impairment and by approximately one half in patients with severe hepatic impairment. | ||
* It is not necessary to modify the | * It is not necessary to modify the sirolimus loading dose in patients with mild, moderate, and severe hepatic impairment. Therapeutic drug monitoring is necessary in all patients with hepatic impairment. | ||
=====Renal Impairment===== | =====Renal Impairment===== | ||
* The effect of renal impairment on the pharmacokinetics of sirolimus is not known. However, there is minimal (2.2%) renal excretion of the drug or its metabolites in healthy volunteers. The loading and the maintenance doses of | * The effect of renal impairment on the pharmacokinetics of sirolimus is not known. However, there is minimal (2.2%) renal excretion of the drug or its metabolites in healthy volunteers. The loading and the maintenance doses of sirolimus need not be adjusted in patients with renal impairment. | ||
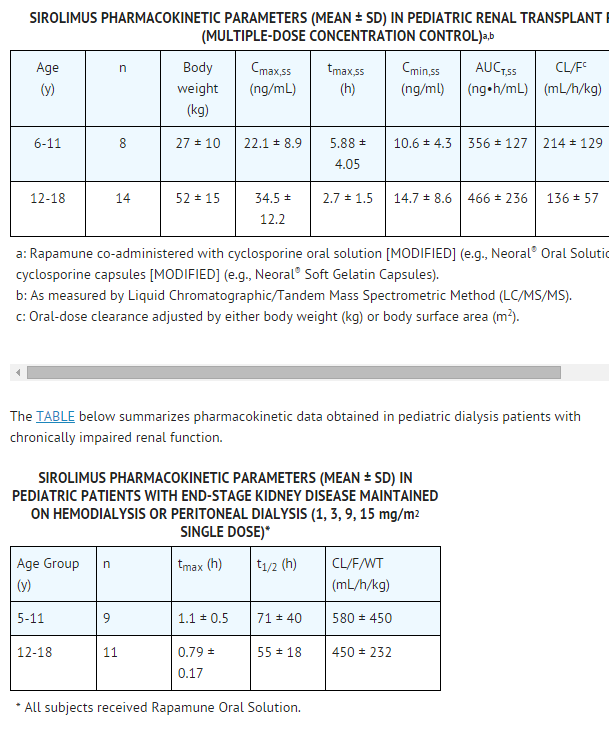
=====Pediatric===== | =====Pediatric===== | ||
* Sirolimus pharmacokinetic data were collected in concentration-controlled trials of pediatric renal transplant patients who were also receiving cyclosporine and corticosteroids. The target ranges for trough concentrations were either 10-20 ng/mL for the 21 children receiving tablets, or 5-15 ng/mL for the one child receiving oral solution. The children aged 6-11 years (n = 8) received mean ± SD doses of 1.75 ± 0.71 mg/day (0.064 ± 0.018 mg/kg, 1.65 ± 0.43 mg/m2). The children aged 12-18 years (n = 14) received mean ± SD doses of 2.79 ± 1.25 mg/day (0.053 ± 0.0150 mg/kg, 1.86± 0.61 mg/m2). At the time of sirolimus blood sampling for pharmacokinetic evaluation, the majority (80%) of these pediatric patients received the | * Sirolimus pharmacokinetic data were collected in concentration-controlled trials of pediatric renal transplant patients who were also receiving cyclosporine and corticosteroids. The target ranges for trough concentrations were either 10-20 ng/mL for the 21 children receiving tablets, or 5-15 ng/mL for the one child receiving oral solution. The children aged 6-11 years (n = 8) received mean ± SD doses of 1.75 ± 0.71 mg/day (0.064 ± 0.018 mg/kg, 1.65 ± 0.43 mg/m2). The children aged 12-18 years (n = 14) received mean ± SD doses of 2.79 ± 1.25 mg/day (0.053 ± 0.0150 mg/kg, 1.86± 0.61 mg/m2). At the time of sirolimus blood sampling for pharmacokinetic evaluation, the majority (80%) of these pediatric patients received the sirolimus dose at 16 hours after the once-daily cyclosporine dose. | ||
: [[File:Sirolimus 06.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 06.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
=====Geriatric===== | =====Geriatric===== | ||
* Clinical studies of | * Clinical studies of sirolimus did not include a sufficient number of patients > 65 years of age to determine whether they will respond differently than younger patients. After the administration of sirolimus Oral Solution or Tablets, sirolimus trough concentration data in renal transplant patients > 65 years of age were similar to those in the adult population 18 to 65 years of age. | ||
=====Gender===== | =====Gender===== | ||
* Sirolimus clearance in males was 12% lower than that in females; male subjects had a significantly longer t1/2 than did female subjects (72.3 hours versus 61.3 hours). Dose adjustments based on gender are not recommended. | * Sirolimus clearance in males was 12% lower than that in females; male subjects had a significantly longer t1/2 than did female subjects (72.3 hours versus 61.3 hours). Dose adjustments based on gender are not recommended. | ||
=====Race===== | =====Race===== | ||
* In the phase 3 trials using | * In the phase 3 trials using sirolimus solution or tablets and cyclosporine oral solution [MODIFIED] (e.g., Neoral® Oral Solution) and/or cyclosporine capsules [MODIFIED] (e.g., Neoral® Soft Gelatin Capsules) [see Clinical Studies (14)], there were no significant differences in mean trough sirolimus concentrations over time between Black (n = 190) and non-Black (n = 852) patients during the first 6 months after transplantation. | ||
=====Drug-Drug Interactions===== | =====Drug-Drug Interactions===== | ||
* Sirolimus is known to be a substrate for both cytochrome CYP3A4 and P-gp. The pharmacokinetic interaction between sirolimus and concomitantly administered drugs is discussed below. Drug interaction studies have not been conducted with drugs other than those described below. | * Sirolimus is known to be a substrate for both cytochrome CYP3A4 and P-gp. The pharmacokinetic interaction between sirolimus and concomitantly administered drugs is discussed below. Drug interaction studies have not been conducted with drugs other than those described below. | ||
=====Cyclosporine===== | =====Cyclosporine===== | ||
* Cyclosporine is a substrate and inhibitor of CYP3A4 and P-gp. Sirolimus should be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and/or cyclosporine capsules (MODIFIED). Sirolimus concentrations may decrease when cyclosporine is discontinued, unless the | * Cyclosporine is a substrate and inhibitor of CYP3A4 and P-gp. Sirolimus should be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and/or cyclosporine capsules (MODIFIED). Sirolimus concentrations may decrease when cyclosporine is discontinued, unless the sirolimus dose is increased. | ||
* In a single-dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg | * In a single-dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg sirolimus Tablets either simultaneously or 4 hours after a 300-mg dose of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). For simultaneous administration, mean Cmax and AUC were increased by 512% and 148%, respectively, relative to administration of sirolimus alone. However, when given 4 hours after cyclosporine administration, sirolimus Cmax and AUC were both increased by only 33% compared with administration of sirolimus alone. | ||
* In a single dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg | * In a single dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg sirolimus Oral Solution either simultaneously or 4 hours after a 300 mg dose of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). For simultaneous administration, the mean Cmax and AUC of sirolimus, following simultaneous administration were increased by 116% and 230%, respectively, relative to administration of sirolimus alone. However, when given 4 hours after Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) administration, sirolimus Cmax and AUC were increased by only 37% and 80%, respectively, compared with administration of sirolimus alone. | ||
* In a single-dose cross-over drug-drug interaction study, 33 healthy volunteers received 5 mg | * In a single-dose cross-over drug-drug interaction study, 33 healthy volunteers received 5 mg sirolimus Oral Solution alone, 2 hours before, and 2 hours after a 300 mg dose of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). When given 2 hours before Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) administration, sirolimus Cmax and AUC were comparable to those with administration of sirolimus alone. However, when given 2 hours after, the mean Cmax and AUC of sirolimus were increased by 126% and 141%, respectively, relative to administration of sirolimus alone. | ||
* Mean cyclosporine Cmax and AUC were not significantly affected when | * Mean cyclosporine Cmax and AUC were not significantly affected when sirolimus Oral Solution was given simultaneously or when administered 4 hours after Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). However, after multiple-dose administration of sirolimus given 4 hours after Neoral® in renal post-transplant patients over 6 months, cyclosporine oral-dose clearance was reduced, and lower doses of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) were needed to maintain target cyclosporine concentration. | ||
* In a multiple-dose study in 150 psoriasis patients, sirolimus 0.5, 1.5, and 3 mg/m2/day was administered simultaneously with Sandimmune® Oral Solution (cyclosporine Oral Solution) 1.25 mg/kg/day. The increase in average sirolimus trough concentrations ranged between 67% to 86% relative to when | * In a multiple-dose study in 150 psoriasis patients, sirolimus 0.5, 1.5, and 3 mg/m2/day was administered simultaneously with Sandimmune® Oral Solution (cyclosporine Oral Solution) 1.25 mg/kg/day. The increase in average sirolimus trough concentrations ranged between 67% to 86% relative to when sirolimus was administered without cyclosporine. The intersubject variability (% CV) for sirolimus trough concentrations ranged from 39.7% to 68.7%. There was no significant effect of multiple-dose sirolimus on cyclosporine trough concentrations following Sandimmune® Oral Solution (cyclosporine oral solution) administration. However, the % CV was higher (range 85.9% - 165%) than those from previous studies. | ||
=====Diltiazem===== | =====Diltiazem===== | ||
* Diltiazem is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 10 mg of sirolimus oral solution and 120 mg of diltiazem to 18 healthy volunteers significantly affected the bioavailability of sirolimus. Sirolimus Cmax, tmax, and AUC were increased 1.4-, 1.3-, and 1.6-fold, respectively. Sirolimus did not affect the pharmacokinetics of either diltiazem or its metabolites desacetyldiltiazem and desmethyldiltiazem. | * Diltiazem is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 10 mg of sirolimus oral solution and 120 mg of diltiazem to 18 healthy volunteers significantly affected the bioavailability of sirolimus. Sirolimus Cmax, tmax, and AUC were increased 1.4-, 1.3-, and 1.6-fold, respectively. Sirolimus did not affect the pharmacokinetics of either diltiazem or its metabolites desacetyldiltiazem and desmethyldiltiazem. | ||
| Line 388: | Line 388: | ||
* Erythromycin is a substrate and inhibitor of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and erythromycin is not recommended. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 800 mg q 8h of erythromycin as erythromycin ethylsuccinate tablets at steady state to 24 healthy volunteers significantly affected the bioavailability of sirolimus and erythromycin. Sirolimus Cmax and AUC were increased 4.4- and 4.2-fold respectively and tmax was increased by 0.4 hr. Erythromycin Cmax and AUC were increased 1.6- and 1.7-fold, respectively, and tmax was increased by 0.3 hr. | * Erythromycin is a substrate and inhibitor of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and erythromycin is not recommended. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 800 mg q 8h of erythromycin as erythromycin ethylsuccinate tablets at steady state to 24 healthy volunteers significantly affected the bioavailability of sirolimus and erythromycin. Sirolimus Cmax and AUC were increased 4.4- and 4.2-fold respectively and tmax was increased by 0.4 hr. Erythromycin Cmax and AUC were increased 1.6- and 1.7-fold, respectively, and tmax was increased by 0.3 hr. | ||
=====Ketoconazole===== | =====Ketoconazole===== | ||
* Ketoconazole is a strong inhibitor of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and ketoconazole is not recommended. Multiple-dose ketoconazole administration significantly affected the rate and extent of absorption and sirolimus exposure after administration of | * Ketoconazole is a strong inhibitor of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and ketoconazole is not recommended. Multiple-dose ketoconazole administration significantly affected the rate and extent of absorption and sirolimus exposure after administration of sirolimus Oral Solution, as reflected by increases in sirolimus Cmax, tmax, and AUC of 4.3-fold, 38%, and 10.9-fold, respectively. However, the terminal t½ of sirolimus was not changed. Single-dose sirolimus did not affect steady-state 12-hour plasma ketoconazole concentrations. | ||
=====Rifampin===== | =====Rifampin===== | ||
* Rifampin is a strong inducer of CYP3A4 and P-gp; co-administration of | * Rifampin is a strong inducer of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and rifampin is not recommended. In patients where rifampin is indicated, alternative therapeutic agents with less enzyme induction potential should be considered. Pretreatment of 14 healthy volunteers with multiple doses of rifampin, 600 mg daily for 14 days, followed by a single 20-mg dose of sirolimus oral solution, greatly decreased sirolimus AUC and Cmax by about 82% and 71%, respectively. | ||
=====Verapamil===== | =====Verapamil===== | ||
* Verapamil is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 180 mg q 12h of verapamil at steady state to 26 healthy volunteers significantly affected the bioavailability of sirolimus and verapamil. Sirolimus Cmax and AUC were increased 2.3- and 2.2-fold, respectively, without substantial change in tmax. The Cmax and AUC of the pharmacologically active S(-) enantiomer of verapamil were both increased 1.5-fold and tmax was decreased by 1.2 hr. | * Verapamil is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 180 mg q 12h of verapamil at steady state to 26 healthy volunteers significantly affected the bioavailability of sirolimus and verapamil. Sirolimus Cmax and AUC were increased 2.3- and 2.2-fold, respectively, without substantial change in tmax. The Cmax and AUC of the pharmacologically active S(-) enantiomer of verapamil were both increased 1.5-fold and tmax was decreased by 1.2 hr. | ||
| Line 404: | Line 404: | ||
:* Sulfamethoxazole/trimethoprim (Bactrim®) | :* Sulfamethoxazole/trimethoprim (Bactrim®) | ||
=====Other Drug-Drug Interactions===== | =====Other Drug-Drug Interactions===== | ||
* Co-administration of | * Co-administration of sirolimus with other known strong inhibitors of CYP3A4 and/or P-gp (such as voriconazole, itraconazole, telithromycin, or clarithromycin) or other known strong inducers of CYP3A4 and/or P-gp (such as rifabutin) is not recommended. In patients in whom strong inhibitors or inducers of CYP3A4 are indicated, alternative therapeutic agents with less potential for inhibition or induction of CYP3A4 should be considered. | ||
* Care should be exercised when drugs or other substances that are substrates and/or inhibitors or inducers of CYP3A4 are administered concomitantly with | * Care should be exercised when drugs or other substances that are substrates and/or inhibitors or inducers of CYP3A4 are administered concomitantly with sirolimus. Other drugs that have the potential to increase sirolimus blood concentrations include (but are not limited to): | ||
* Calcium channel blockers | * Calcium channel blockers | ||
:* Nicardipine. | :* Nicardipine. | ||
| Line 422: | Line 422: | ||
:* Rifapentine. | :* Rifapentine. | ||
=====Other Drug-Food Interactions===== | =====Other Drug-Food Interactions===== | ||
* Grapefruit juice reduces CYP3A4-mediated drug metabolism. Grapefruit juice must not be taken with or used for dilution of | * Grapefruit juice reduces CYP3A4-mediated drug metabolism. Grapefruit juice must not be taken with or used for dilution of sirolimus. | ||
=====Drug-Herb Interactions===== | =====Drug-Herb Interactions===== | ||
* St. John's Wort (hypericum perforatum) induces CYP3A4 and P-gp. Since sirolimus is a substrate for both cytochrome CYP3A4 and P-gp, there is the potential that the use of St. John's Wort in patients receiving | * St. John's Wort (hypericum perforatum) induces CYP3A4 and P-gp. Since sirolimus is a substrate for both cytochrome CYP3A4 and P-gp, there is the potential that the use of St. John's Wort in patients receiving sirolimus could result in reduced sirolimus concentrations. | ||
|nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | ||
* Carcinogenicity studies were conducted in mice and rats. In an 86-week female mouse study at sirolimus doses 30 to 120 times higher than the 2 mg daily clinical dose (adjusted for body surface area), there was a statistically significant increase in malignant lymphoma at all dose levels compared with controls. | * Carcinogenicity studies were conducted in mice and rats. In an 86-week female mouse study at sirolimus doses 30 to 120 times higher than the 2 mg daily clinical dose (adjusted for body surface area), there was a statistically significant increase in malignant lymphoma at all dose levels compared with controls. | ||
| Line 431: | Line 431: | ||
* Fertility was diminished slightly in both male and female rats following oral administration of sirolimus at doses approximately 10 times or 2 times, respectively, the clinical dose of 2 mg daily (adjusted for body surface area). In male rats, atrophy of testes, epididymides, prostate, seminiferous tubules and/or reduction in sperm counts were observed. In female rats, reduced size of ovaries and uteri was observed. Reduction of sperm count in male rats was reversible upon cessation of dosing in one study. Testicular tubular degeneration was also seen in a 4‑week intravenous study of sirolimus in monkeys at doses that were approximately equal to the clinical dose (adjusted for body surface area). | * Fertility was diminished slightly in both male and female rats following oral administration of sirolimus at doses approximately 10 times or 2 times, respectively, the clinical dose of 2 mg daily (adjusted for body surface area). In male rats, atrophy of testes, epididymides, prostate, seminiferous tubules and/or reduction in sperm counts were observed. In female rats, reduced size of ovaries and uteri was observed. Reduction of sperm count in male rats was reversible upon cessation of dosing in one study. Testicular tubular degeneration was also seen in a 4‑week intravenous study of sirolimus in monkeys at doses that were approximately equal to the clinical dose (adjusted for body surface area). | ||
|clinicalStudies=====Prophylaxis of Organ Rejection==== | |clinicalStudies=====Prophylaxis of Organ Rejection==== | ||
===== | =====Sirolimus Oral Solution===== | ||
* The safety and efficacy of | * The safety and efficacy of sirolimus Oral Solution for the prevention of organ rejection following renal transplantation were assessed in two randomized, double-blind, multicenter, controlled trials. These studies compared two dose levels of sirolimus Oral Solution (2 mg and 5 mg, once daily) with azathioprine (Study 1) or placebo (Study 2) when administered in combination with cyclosporine and corticosteroids. Study 1 was conducted in the United States at 38 sites. Seven hundred nineteen (719) patients were enrolled in this trial and randomized following transplantation; 284 were randomized to receive sirolimus Oral Solution 2 mg/day; 274 were randomized to receive sirolimus Oral Solution 5 mg/day, and 161 to receive azathioprine 2-3 mg/kg/day. Study 2 was conducted in Australia, Canada, Europe, and the United States, at a total of 34 sites. Five hundred seventy-six (576) patients were enrolled in this trial and randomized before transplantation; 227 were randomized to receive sirolimus Oral Solution 2 mg/day; 219 were randomized to receive sirolimus Oral Solution 5 mg/day, and 130 to receive placebo. In both studies, the use of antilymphocyte antibody induction therapy was prohibited. In both studies, the primary efficacy endpoint was the rate of efficacy failure in the first 6 months after transplantation. Efficacy failure was defined as the first occurrence of an acute rejection episode (confirmed by biopsy), graft loss, or death. | ||
* The TABLES below summarize the results of the primary efficacy analyses from these trials. | * The TABLES below summarize the results of the primary efficacy analyses from these trials. Sirolimus Oral Solution, at doses of 2 mg/day and 5 mg/day, significantly reduced the incidence of efficacy failure (statistically significant at the< 0.025 level; nominal significance level adjusted for multiple [2] dose comparisons) at 6 months following transplantation compared with both azathioprine and placebo. | ||
: [[File:Sirolimus07.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus07.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* The reduction in the incidence of first biopsy-confirmed acute rejection episodes in patients treated with | * The reduction in the incidence of first biopsy-confirmed acute rejection episodes in patients treated with sirolimus compared with the control groups included a reduction in all grades of rejection. | ||
* In Study 1, which was prospectively stratified by race within center, efficacy failure was similar for | * In Study 1, which was prospectively stratified by race within center, efficacy failure was similar for sirolimus Oral Solution 2 mg/day and lower for sirolimus Oral Solution 5 mg/day compared with azathioprine in Black patients. In Study 2, which was not prospectively stratified by race, efficacy failure was similar for both sirolimus Oral Solution doses compared with placebo in Black patients. The decision to use the higher dose of sirolimus Oral Solution in Black patients must be weighed against the increased risk of dose-dependent adverse events that were observed with the sirolimus Oral Solution 5-mg dose. | ||
: [[File:Sirolimus 08.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 08.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Mean glomerular filtration rates (GFR) post-transplant were calculated by using the Nankivell equation at 12 and 24 months for Study 1, and 12 and 36 months for Study 2. Mean GFR was lower in patients treated with cyclosporine and | * Mean glomerular filtration rates (GFR) post-transplant were calculated by using the Nankivell equation at 12 and 24 months for Study 1, and 12 and 36 months for Study 2. Mean GFR was lower in patients treated with cyclosporine and sirolimus Oral Solution compared with those treated with cyclosporine and the respective azathioprine or placebo control. | ||
: [[File:Sirolimus 09.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 09.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Within each treatment group in Studies 1 and 2, mean GFR at one-year post-transplant was lower in patients who experienced at least one episode of biopsy-proven acute rejection, compared with those who did not. | * Within each treatment group in Studies 1 and 2, mean GFR at one-year post-transplant was lower in patients who experienced at least one episode of biopsy-proven acute rejection, compared with those who did not. | ||
* Renal function should be monitored, and appropriate adjustment of the immunosuppressive regimen should be considered in patients with elevated or increasing serum creatinine levels. | * Renal function should be monitored, and appropriate adjustment of the immunosuppressive regimen should be considered in patients with elevated or increasing serum creatinine levels. | ||
===== | =====Sirolimus Tablets===== | ||
* The safety and efficacy of | * The safety and efficacy of sirolimus Oral Solution and sirolimus tablets for the prevention of organ rejection following renal transplantation were demonstrated to be clinically equivalent in a randomized, multicenter, controlled trial. | ||
=====Cyclosporine Withdrawal Study===== | =====Cyclosporine Withdrawal Study===== | ||
* The safety and efficacy of | * The safety and efficacy of sirolimus as a maintenance regimen were assessed following cyclosporine withdrawal at 3 to 4 months after renal transplantation. Study 3 was a randomized, multicenter, controlled trial conducted at 57 centers in Australia, Canada, and Europe. Five hundred twenty-five (525) patients were enrolled. All patients in this study received the tablet formulation. | ||
* This study compared patients who were administered | * This study compared patients who were administered sirolimus, cyclosporine, and corticosteroids continuously with patients who received this same standardized therapy for the first 3 months after transplantation (pre-randomization period) followed by the withdrawal of [[cyclosporine]]. During cyclosporine withdrawal, the sirolimus dosages were adjusted to achieve targeted sirolimus whole blood trough concentration ranges (16 to 24 ng/mL until month 12, then 12 to 20 ng/mL thereafter, expressed as chromatographic assay values). At 3 months, 430 patients were equally randomized to either continue sirolimus with cyclosporine therapy or to receive sirolimus as a maintenance regimen following cyclosporine withdrawal. | ||
* Eligibility for randomization included no Banff Grade 3 acute rejection or vascular rejection episode in the 4 weeks before random assignment, serum creatinine ≤ 4.5 mg/dL, and adequate renal function to support cyclosporine withdrawal (in the opinion of the investigator). The primary efficacy endpoint was graft survival at 12 months after transplantation. Secondary efficacy endpoints were the rate of biopsy-confirmed acute rejection, patient survival, incidence of efficacy failure (defined as the first occurrence of either biopsy-proven acute rejection, graft loss, or death), and treatment failure (defined as the first occurrence of either discontinuation, acute rejection, graft loss, or death). | * Eligibility for randomization included no Banff Grade 3 [[acute rejection]] or vascular rejection episode in the 4 weeks before random assignment, serum creatinine ≤ 4.5 mg/dL, and adequate renal function to support cyclosporine withdrawal (in the opinion of the investigator). The primary efficacy endpoint was graft survival at 12 months after transplantation. Secondary efficacy endpoints were the rate of biopsy-confirmed acute rejection, patient survival, incidence of efficacy failure (defined as the first occurrence of either biopsy-proven acute rejection, graft loss, or death), and treatment failure (defined as the first occurrence of either discontinuation, acute rejection, graft loss, or death). | ||
* The following TABLE summarizes the resulting graft and patient survival at 12, 24, and 36 months for this trial. At 12, 24, and 36 months, graft and patient survival were similar for both groups. | * The following TABLE summarizes the resulting graft and patient survival at 12, 24, and 36 months for this trial. At 12, 24, and 36 months, graft and patient survival were similar for both groups. | ||
: [[File:Sirolimus 10.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 10.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
: [[File:Sirolimus 11.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 11.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* The mean GFR at 12, 24, and 36 months, calculated by the Nankivell equation, was significantly higher for patients receiving | * The mean GFR at 12, 24, and 36 months, calculated by the Nankivell equation, was significantly higher for patients receiving sirolimus as a maintenance regimen following [[cyclosporine]] withdrawal than for those in the sirolimus with [[cyclosporine]] therapy group. Patients who had an acute rejection prior to randomization had a significantly higher GFR following [[cyclosporine]] withdrawal compared to those in the sirolimus with [[cyclosporine]] group. There was no significant difference in GFR between groups for patients who experienced acute rejection post-randomization. | ||
* Although the initial protocol was designed for 36 months, there was a subsequent amendment to extend this study. The results for the cyclosporine withdrawal group at months 48 and 60 were consistent with the results at month 36. Fifty-two percent (112/215) of the patients in the | * Although the initial protocol was designed for 36 months, there was a subsequent amendment to extend this study. The results for the cyclosporine withdrawal group at months 48 and 60 were consistent with the results at month 36. Fifty-two percent (112/215) of the patients in the sirolimus with [[cyclosporine]] withdrawal group remained on therapy to month 60 and showed sustained GFR. | ||
=====High-Immunologic Risk Patients===== | =====High-Immunologic Risk Patients===== | ||
* | * Sirolimus was studied in a one-year, clinical trial in high risk patients (Study 4) who were defined as Black [[transplant]] recipients and/or repeat renal transplant recipients who lost a previous allograft for immunologic reasons and/or patients with high panel-reactive antibodies (PRA; peak PRA level > 80%). Patients received concentration-controlled sirolimus and [[cyclosporine]] (MODIFIED), and [[corticosteroids]] per local practice. The sirolimus dose was adjusted to achieve target whole [[blood]] trough sirolimus concentrationsof 10-15 ng/mL (chromatographic method) throughout the 12-month study period. The cyclosporine dose was adjusted to achieve target whole blood trough concentrations of 200-300 ng/mL through week 2, 150-200 ng/mL from week 2 to week 26, and 100-150 ng/mL from week 26 to week 52 for the observed trough concentrations ranges. Antibody induction was allowed per protocol as prospectively defined at each transplant center, and was used in 88.4% of patients. The study was conducted at 35 centers in the United States. | ||
* A total of 224 patients received a transplant and at least one dose of sirolimus and cyclosporine and was comprised of 77.2% Black patients, 24.1% repeat renal transplant recipients, and 13.5% patients with high PRA. Efficacy was assessed with the following endpoints, measured at 12 months: efficacy failure (defined as the first occurrence of biopsy-confirmed acute rejection, graft loss, or death), first occurrence of graft loss or death, and renal function as measured by the calculated GFR using the Nankivell formula. The TABLE below summarizes the result of these endpoints. | * A total of 224 patients received a transplant and at least one dose of sirolimus and cyclosporine and was comprised of 77.2% Black patients, 24.1% repeat renal transplant recipients, and 13.5% patients with high PRA. Efficacy was assessed with the following endpoints, measured at 12 months: efficacy failure (defined as the first occurrence of biopsy-confirmed acute rejection, graft loss, or death), first occurrence of graft loss or death, and renal function as measured by the calculated GFR using the Nankivell formula. The TABLE below summarizes the result of these endpoints. | ||
: [[File:Sirolimus 12.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 12.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Patient survival at 12 months was 94.6%. The incidence of biopsy-confirmed acute rejection was 17.4% and the majority of the episodes of acute rejection were mild in severity. | * Patient survival at 12 months was 94.6%. The incidence of biopsy-confirmed acute rejection was 17.4% and the majority of the episodes of acute rejection were mild in severity. | ||
=====Conversion from Calcineurin Inhibitors to | =====Conversion from Calcineurin Inhibitors to Sirolimus in Maintenance Renal Transplant Patients===== | ||
* Conversion from calcineurin inhibitors (CNI) to | * Conversion from calcineurin inhibitors (CNI) to sirolimus was assessed in maintenance [[renal transplant]] patients 6 months to 10 years post‑transplant (Study 5). This study was a randomized, multicenter, controlled trial conducted at 111 centers globally, including US and Europe, and was intended to show that [[renal]] function was improved by conversion from CNI to sirolimus. Eight hundred thirty (830) patients were enrolled and stratified by baseline calculated glomerular filtration rate (GFR, 20-40 mL/min vs. greater than 40 mL/min). In this trial there was no benefit associated with conversion with regard to improvement in [[renal]] function and a greater incidence of [[proteinuria]] in the sirolimus conversion arm. In addition, enrollment of patients with baseline calculated GFR less than 40 mL/min was discontinued due to a higher rate of serious adverse events, including pneumonia, acute rejection, graft loss and death. | ||
* This study compared renal transplant patients (6-120 months after transplantation) who were converted from calcineurin inhibitors to | * This study compared renal transplant patients (6-120 months after [[transplantation]]) who were converted from [[calcineurin inhibitors]] to sirolimus, with patients who continued to receive calcineurin inhibitors. Concomitant immunosuppressive medications included [[mycophenolate mofetil]] (MMF), [[azathioprine]] (AZA), and [[corticosteroids]]. sirolimus was initiated with a single loading dose of 12-20 mg, after which dosing was adjusted to achieve a target sirolimus whole blood trough concentration of 8-20 ng/mL (chromatographic method). The efficacy endpoint was calculated GFR at 12 months post-randomization. Additional endpoints included biopsy-confirmed acute rejection, graft loss, and death. Findings in the patient stratum with baseline calculated GFR greater than 40 mL/min (sirolimus conversion, n = 497; CNI continuation, n = 246) are summarized BELOW: There was no clinically or statistically significant improvement in Nankivell GFR compared to baseline. | ||
: [[File:Sirolimus 13.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 13.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* The rates of acute rejection, graft loss, and death were similar at 1 and 2 years. Treatment-emergent adverse events occurred more frequently during the first 6 months after | * The rates of acute rejection, graft loss, and death were similar at 1 and 2 years. Treatment-emergent adverse events occurred more frequently during the first 6 months after sirolimus conversion. The rates of [[pneumonia]] were significantly higher for the sirolimus conversion group. | ||
* While the mean and median values for urinary protein to creatinine ratio were similar between treatment groups at baseline, significantly higher mean and median levels of urinary protein excretion were seen in the | * While the mean and median values for urinary [[protein]] to creatinine ratio were similar between treatment groups at baseline, significantly higher mean and median levels of urinary protein excretion were seen in the sirolimus conversion arm at 1 year and at 2 years, as shown in the TABLE below. | ||
* In addition, when compared to patients who continued to receive calcineurin inhibitors, a higher percentage of patients had urinary protein to creatinine ratios > 1 at 1 and 2 years after sirolimus conversion. This difference was seen in both patients who had a urinary protein to creatinine ratio ≤ 1 and those who had a protein to creatinine ratio > 1 at baseline. More patients in the sirolimus conversion group developed nephrotic range proteinuria, as defined by a urinary protein to creatinine ratio > 3.5 (46/482 [9.5%] vs. 9/239 [3.8%]), even when the patients with baseline nephrotic range proteinuria were excluded. The rate of nephrotic range proteinuria was significantly higher in the sirolimus conversion group compared to the calcineurin inhibitor continuation group with baseline urinary protein to creatinine ratio > 1 (13/29 vs. 1/14), excluding patients with baseline nephrotic range proteinuria. | * In addition, when compared to patients who continued to receive calcineurin inhibitors, a higher percentage of patients had urinary protein to creatinine ratios > 1 at 1 and 2 years after sirolimus conversion. This difference was seen in both patients who had a urinary protein to creatinine ratio ≤ 1 and those who had a [[protein]] to creatinine ratio > 1 at baseline. More patients in the sirolimus conversion group developed nephrotic range [[proteinuria]], as defined by a urinary protein to creatinine ratio > 3.5 (46/482 [9.5%] vs. 9/239 [3.8%]), even when the patients with baseline nephrotic range proteinuria were excluded. The rate of nephrotic range proteinuria was significantly higher in the sirolimus conversion group compared to the [[calcineurin]] inhibitor continuation group with baseline urinary protein to creatinine ratio > 1 (13/29 vs. 1/14), excluding patients with baseline nephrotic range [[proteinuria]]. | ||
: [[File:Sirolimus 14.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sirolimus 14.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* The above information should be taken into account when considering conversion from calcineurin inhibitors to | * The above information should be taken into account when considering conversion from calcineurin inhibitors to sirolimus in stable renal transplant patients due to the lack of evidence showing that renal function improves following conversion, and the finding of a greater increment in urinary protein excretion, and an increased incidence of treatment-emergent nephrotic range [[proteinuria]] following conversion to sirolimus. | ||
* This was particularly true among patients with existing abnormal urinary protein excretion prior to conversion. | * This was particularly true among patients with existing abnormal urinary protein excretion prior to conversion. | ||
=====Pediatrics===== | =====Pediatrics===== | ||
* | * Sirolimus was evaluated in a 36-month, open-label, randomized, controlled clinical trial at 14 North American centers in pediatric (aged 3 to < 18 years) renal transplant patients considered to be at high-immunologic risk for developing chronic [[allograft]] [[nephropathy]], defined as a history of one or more acute [[allograft]] rejection episodes and/or the presence of chronic allograft nephropathy on a [[renal biopsy]]. Seventy-eight (78) subjects were randomized in a 2:1 ratio to sirolimus (sirolimus target concentrations of 5 to 15 ng/mL, by chromatographic assay, n = 53) in combination with a calcineurin inhibitor and corticosteroids or to continue calcineurin-inhibitor-based immunosuppressive therapy (n = 25). The primary endpoint of the study was efficacy failure as defined by the first occurrence of biopsy-confirmed acute rejection, graft loss, or death, and the trial was designed to show superiority of sirolimus added to a [[calcineurin]]-inhibitor-based immunosuppressive regimen compared to a [[calcineurin]]-inhibitor-based regimen. | ||
* The cumulative incidence of efficacy failure up to 36 months was 45.3% in the | * The cumulative incidence of efficacy failure up to 36 months was 45.3% in the sirolimus group compared to 44.0% in the control group, and did not demonstrate superiority. There was one death in each group. The use of sirolimus in combination with [[calcineurin]] inhibitors and corticosteroids was associated with an increased risk of deterioration of renal function, serum lipid abnormalities (including, but not limited to, increased serum triglycerides and cholesterol), and urinary tract infections . This study does not support the addition of sirolimus to calcineurin-inhibitor-based immunosuppressive therapy in this subpopulation of pediatric renal transplant patients. | ||
|howSupplied=* Since | |howSupplied=* Since sirolimus is not absorbed through the skin, there are no special precautions. However, if direct contact with the [[skin]] or [[mucous membranes]] occurs, wash thoroughly with soap and water; rinse eyes with plain water. | ||
===== | =====Sirolimus Oral Solution===== | ||
* Each | * Each sirolimus Oral Solution carton, NDC 0008-1030-06, contains one 2 oz (60 mL fill) amber glass bottle of sirolimus (concentration of 1 mg/mL), one oral syringe adapter for fitting into the neck of the bottle, sufficient disposable amber oral syringes and caps for daily dosing, and a carrying case. | ||
* | * Sirolimus Oral Solution bottles should be stored protected from light and refrigerated at 2°C to 8°C (36°F to 46°F). Once the bottle is opened, the contents should be used within one month. If necessary, the patient may store the bottles at room temperatures up to 25°C (77°F) for a short period of time (e.g., not more than 15 days for the bottles). | ||
* An amber syringe and cap are provided for dosing, and the product may be kept in the syringe for a maximum of 24 hours at room temperatures up to 25°C (77°F) or refrigerated at 2°C to 8°C (36°F to 46°F). The syringe should be discarded after one use. After dilution, the preparation should be used immediately. | * An amber syringe and cap are provided for dosing, and the product may be kept in the syringe for a maximum of 24 hours at room temperatures up to 25°C (77°F) or refrigerated at 2°C to 8°C (36°F to 46°F). The syringe should be discarded after one use. After dilution, the preparation should be used immediately. | ||
* | * Sirolimus Oral Solution provided in bottles may develop a slight haze when refrigerated. If such a haze occurs, allow the product to stand at room temperature and shake gently until the haze disappears. The presence of this haze does not affect the quality of the product. | ||
===== | =====Sirolimus Tablets===== | ||
* | * Sirolimus Tablets are available as follows: | ||
* NDC 0008-1041-05, 1 mg, white, triangular-shaped tablets | * NDC 0008-1041-05, 1 mg, white, triangular-shaped tablets marked"Rapamune 1 mg” on one side; bottle containing 100 tablets. | ||
* NDC 0008-1041-10, 1 mg, white, triangular-shaped tablets marked“RAPAMUNE 1 mg” on one side; in Redipak® cartons of 100 tablets (10 blister cards of 10 tablets each). | * NDC 0008-1041-10, 1 mg, white, triangular-shaped tablets marked“RAPAMUNE 1 mg” on one side; in Redipak® cartons of 100 tablets (10 blister cards of 10 tablets each). | ||
* NDC 0008-1042-05, 2 mg, yellow-to-beige triangular-shaped tablets marked | * NDC 0008-1042-05, 2 mg, yellow-to-beige triangular-shaped tablets marked “ Rapamune 2 mg” on one side; bottle containing 100 tablets. | ||
|storage=* Rapamune Tablets should be stored at 20° to 25°C [USP Controlled Room Temperature] (68° to 77°F). Use cartons to protect blister cards and strips from light. Dispense in a tight, light-resistant container as defined in the USP. | |storage=* Rapamune Tablets should be stored at 20° to 25°C [USP Controlled Room Temperature] (68° to 77°F). Use cartons to protect blister cards and strips from light. Dispense in a tight, light-resistant container as defined in the USP. | ||
|packLabel=<!--Patient Counseling Information--> | |packLabel=<!--Patient Counseling Information--> | ||
| Line 487: | Line 487: | ||
* Patients should be given complete dosage instructions. | * Patients should be given complete dosage instructions. | ||
=====Skin Cancer Events===== | =====Skin Cancer Events===== | ||
* Patients should be told that exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor because of the increased risk for skin cancer. | * Patients should be told that exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor because of the increased risk for [[skin]] [[cancer]]. | ||
=====Pregnancy Risks===== | =====Pregnancy Risks===== | ||
* Women of childbearing potential should be informed of the potential risks during pregnancy and told that they should use effective contraception prior to initiation of | * Women of childbearing potential should be informed of the potential risks during pregnancy and told that they should use effective contraception prior to initiation of sirolimus therapy, during sirolimus therapy, and for 12 weeks after sirolimus therapy has been stopped. | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=*Rapamune ®<ref>{{Cite web | title = RAPAMUNE- sirolimus tablet, sugar coated | url=http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0f26220a-f4ab-4ceb-9a98-a13dbdeed81f&#section-2.3 }}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
Revision as of 15:32, 13 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
IMMUNOSUPPRESSION, EXCESS MORTALITY IN DE NOVO LIVER TRANSPLANTATION, AND BRONCHIAL ANASTOMOTIC DEHISCENCE
See full prescribing information for complete Boxed Warning.
INCREASED SUSCEPTIBILITY TO INFECTION AND THE POSSIBLE DEVELOPMENT OF LYMPHOMA AND OTHER MALIGNANCIES MAY RESULT FROM IMMUNOSUPPRESSION
LIVER TRANSPLANTATION– EXCESS MORTALITY, GRAFT LOSS, AND HEPATIC ARTERY THROMBOSIS (HAT)
LUNG TRANSPLANTATION– BRONCHIAL ANASTOMOTIC DEHISCENCE
|
Overview
Sirolimus is a immunomodulatory drug that is FDA approved for the prophylaxis of organ rejection in renal transplantation. There is a Black Box Warning for this drug as shown here. Common adverse reactions include lymphedema, pericardial effusion, hepatotoxicity, hypersensitivity, tuberculosis, interstitial lung disease, exfoliative dermatitis, nephrotic syndrome.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Organ Rejection in Renal Transplantation
- Sirolimus is indicated for the prophylaxis of organ rejection in patients aged 13 years or older receiving renal transplants. Therapeutic drug monitoring is recommended for all patients receiving sirolimus.
- In patients at low- to moderate-immunologic risk, it is recommended that sirolimus be used initially in a regimen with cyclosporine and corticosteroids; cyclosporine should be withdrawn 2 to 4 months after transplantation.
- In patients at high-immunologic risk (defined as Black recipients and/or repeat renal transplant recipients who lost a previous allograft for immunologic reason and/or patients with high panel-reactive antibodies [PRA; peak PRA level > 80%]), it is recommended that sirolimus be used in combination with cyclosporine and corticosteroids for the first year following transplantation.
Limitations of Use
- Cyclosporine withdrawal has not been studied in patients with Banff Grade 3 acute rejection or vascular rejection prior to cyclosporine withdrawal, those who are dialysis-dependent, those with serum creatinine > 4.5 mg/dL, Black patients, patients of multi-organ transplants, secondary transplants, or those with high levels of panel-reactive antibodies.
- In patients at high-immunologic risk, the safety and efficacy of sirolimus used in combination with cyclosporine and corticosteroids has not been studied beyond one year; therefore after the first 12 months following transplantation, any adjustments to the immunosuppressive regimen should be considered on the basis of the clinical status of the patient.
- In pediatric patients, the safety and efficacy of sirolimus have not been established in patients < 13 years old, or in pediatric (< 18 years) renal transplant patients considered at high-immunologic risk.
- The safety and efficacy of de novo use of sirolimus without cyclosporine have not been established in renal transplant patients.
- The safety and efficacy of conversion from calcineurin inhibitors to Rapamune in maintenance renal transplant patients have not been established.
- Sirolimus is to be administered orally once daily, consistently with or without food.
- Tablets should not be crushed, chewed or split. Patients unable to take the tablets should be prescribed the solution and instructed in its use.
- The initial dose of sirolimus should be administered as soon as possible after transplantation. It is recommended that sirolimus be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and or/cyclosporine capsules (MODIFIED).
- Frequent sirolimus dose adjustments based on non-steady-state sirolimus concentrations can lead to overdosing or underdosing because sirolimus has a long half-life.
- Once sirolimus maintenance dose is adjusted, patients should continue on the new maintenance dose for at least 7 to 14 days before further dosage adjustment with concentration monitoring. In most patients, dose adjustments can be based on simple proportion: new sirolimus dose = current dose x (target concentration/current concentration).
- A loading dose should be considered in addition to a new maintenance dose when it is necessary to increase sirolimus trough concentrations: sirolimus loading dose = 3 x (new maintenance dose - current maintenance dose).
- The maximum sirolimus dose administered on any day should not exceed 40 mg. If an estimated daily dose exceeds 40 mg due to the addition of a loading dose, the loading dose should be administered over 2 days.
- Sirolimus trough concentrations should be monitored at least 3 to 4 days after a loading dose(s).
- Two milligrams (2 mg) of Sirolimus Oral Solution have been demonstrated to be clinically equivalent to 2 mg sirolimus Tablets; hence, are interchangeable on a mg-to-mg basis.
- However, it is not known if higher doses of sirolimus Oral Solution are clinically equivalent to higher doses of sirolimus Tablets on a mg‑to‑mg basis.
Patients at Low- to Moderate-Immunologic Risk
Sirolimus and Cyclosporine Combination Therapy
- For de novo renal transplant patients, it is recommended that sirolimus Oral Solution and Tablets be used initially in a regimen with cyclosporine and corticosteroids.
- A loading dose of sirolimus equivalent to 3 times the maintenance dose should be given, i.e. a daily maintenance dose of 2 mg should be preceded with a loading dose of 6 mg. Therapeutic drug monitoring should be used to maintain sirolimus drug concentrations within the target-range.
Sirolimus Following Cyclosporine Withdrawal
- At 2 to 4 months following transplantation, cyclosporine should be progressively discontinued over 4 to 8 weeks, and the sirolimus dose should be adjusted to obtain sirolimus whole blood trough concentrations within the target-range.
- Because cyclosporine inhibits the metabolism and transport of sirolimus, sirolimus concentrations may decrease when cyclosporine is discontinued, unless the sirolimus dose is increased.
Patients at High-Immunologic Risk
- In patients with high-immunologic risk, it is recommended that sirolimus be used in combination with cyclosporine and corticosteroids for the first 12 months following transplantation. * The safety and efficacy of this combination in high-immunologic risk patients has not been studied beyond the first 12 months. Therefore, after the first 12 months following transplantation, any adjustments to the immunosuppressive regimen should be considered on the basis of the clinical status of the patient.
- For patients receiving sirolimus with cyclosporine, sirolimus therapy should be initiated with a loading dose of up to 15 mg on day 1 post-transplantation. Beginning on day 2, an initial maintenance dose of 5 mg/day should be given. A trough level should be obtained between days 5 and 7, and the daily dose of sirolimus should thereafter be adjusted.
- The starting dose of cyclosporine should be up to 7 mg/kg/day in divided doses and the dose should subsequently be adjusted to achieve target whole blood trough concentrations. Prednisone should be administered at a minimum of 5 mg/day.
- Antibody induction therapy may be used.
Therapeutic Drug Monitoring
- Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the sirolimus dosage form is made, and during concurrent administration of strong CYP3A4 inducers and inhibitors.
- Therapeutic drug monitoring should not be the sole basis for adjusting sirolimus therapy. Careful attention should be made to clinical signs/symptoms, tissue biopsy findings, and laboratory parameters.
- When used in combination with cyclosporine, sirolimus trough concentrations should be maintained within the target-range.
- Following cyclosporine withdrawal in transplant patients at low- to moderate-immunologic risk, the target sirolimus trough concentrations should be 16 to 24 ng/mL for the first year following transplantation. Thereafter, the target sirolimus concentrations should be 12 to 20 ng/mL.
- The above recommended 24-hour trough concentration ranges for sirolimus are based on chromatographic methods. On average, chromatographic methods (HPLC UV or LC/MS/MS) yield results that are approximately 20% lower than the immunoassay for whole blood concentration determinations.
- Currently in clinical practice, sirolimus whole blood concentrations are being measured by both chromatographic and immunoassay methodologies. Because the measured sirolimus whole blood concentrations depend on the type of assay used, the concentrations obtained by these different methodologies are not interchangeable.
- Adjustments to the targeted range should be made according to the assay utilized to determine sirolimus trough concentrations. A discussion of different assay methods is contained in Clinical Therapeutics, Volume 22, Supplement B, April 2000.
Patients with Low Body Weight
- The initial dosage in patients ≥ 13 years who weigh less than 40 kg should be adjusted, based on body surface area, to 1 mg/m2/day. The loading dose should be 3 mg/m2.
Patients with Hepatic Impairment
- It is recommended that the maintenance dose of sirolimus be reduced by approximately one third in patients with mild or moderate hepatic impairment and by approximately one half in patients with severe hepatic impairment. It is not necessary to modify the sirolimus loading dose.
Patients with Renal Impairment
- Dosage adjustment is not needed in patients with impaired renal function.
Instructions for Dilution and Administration of sirolimus Oral Solution
- The amber oral dose syringe should be used to withdraw the prescribed amount of sirolimus Oral Solution from the bottle. Empty the correct amount of sirolimus from the syringe into only a glass or plastic container holding at least two (2) ounces (1/4 cup, 60 mL) of water or orange juice. No other liquids, including grapefruit juice, should be used for dilution. Stir vigorously and drink at once. Refill the container with an additional volume [minimum of four (4) ounces (1/2 cup, 120 mL)] of water or orange juice, stir vigorously, and drink at once.
- Sirolimus Oral Solution contains polysorbate 80, which is known to increase the rate of di‑(2‑ethylhexyl)phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of sirolimus Oral Solution. It is important that these recommendations be followed closely.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sirolimus in adult patients.
Non–Guideline-Supported Use
- Renal transplant rejection.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Sirolimus in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sirolimus in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sirolimus in pediatric patients.
Contraindications
- Sirolimus is contraindicated in patients with a hypersensitivity to sirolimus.
Warnings
|
IMMUNOSUPPRESSION, EXCESS MORTALITY IN DE NOVO LIVER TRANSPLANTATION, AND BRONCHIAL ANASTOMOTIC DEHISCENCE
See full prescribing information for complete Boxed Warning.
INCREASED SUSCEPTIBILITY TO INFECTION AND THE POSSIBLE DEVELOPMENT OF LYMPHOMA AND OTHER MALIGNANCIES MAY RESULT FROM IMMUNOSUPPRESSION
LIVER TRANSPLANTATION– EXCESS MORTALITY, GRAFT LOSS, AND HEPATIC ARTERY THROMBOSIS (HAT)
LUNG TRANSPLANTATION– BRONCHIAL ANASTOMOTIC DEHISCENCE
|
Increased Susceptibility to Infection and the Possible Development of Lymphoma
- Increased susceptibility to infection and the possible development of lymphoma and other malignancies, particularly of the skin, may result from immunosuppression. The rates of lymphoma/lymphoproliferative disease observed in Studies 1 and 2 were 0.7-3.2% (for sirolimus-treated patients) versus 0.6-0.8% (azathioprine and placebo control).
- Oversuppression of the immune system can also increasesusceptibility to infection, including opportunistic infections such as tuberculosis, fatal infections, and sepsis. Only physicians experienced in immunosuppressive therapy and management of organ transplant patients should use sirolimus. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
Liver Transplantation – Excess Mortality, Graft Loss, and Hepatic Artery Thrombosis (HAT)
- The use of sirolimus in combination with tacrolimus was associated with excess mortality and graft loss in a study in de novo liver transplant patients (22% in combination versus 9% on tacrolimus alone). Many of these patients had evidence of infection at or near the time of death.
- In this and another study in de novo liver transplant patients, the use of sirolimus in combination with cyclosporine or tacrolimus was associated with an increase in HAT (7% in combination versus 2% in the control arm); most cases of HAT occurred within 30 days post-transplantation, and most led to graft loss or death.
- The safety and efficacy of sirolimus as immunosuppressive therapy have not been established in liver transplant patients; therefore, such use is not recommended.
Lung Transplantation – Bronchial Anastomotic Dehiscence
- Cases of bronchial anastomotic dehiscence, most fatal, have been reported in de novo lung transplant patients when sirolimus has been used as part of an immunosuppressive regimen.
- The safety and efficacy of sirolimus as immunosuppressive therapy have not been established in lung transplant patients; therefore, such use is not recommended.
Hypersensitivity Reactions
- Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, exfoliative dermatitis and hypersensitivity vasculitis, have been associated with the administration of sirolimus.
Angioedema
- Sirolimus has been associated with the development of angioedema. The concomitant use of sirolimus with other drugs known to cause angioedema, such as ACE-inhibitors, may increase the risk of developing angioedema.
Fluid Accumulation and Wound Healing
- There have been reports of impaired or delayed wound healing in patients receiving sirolimus, including lymphocele and wound dehiscence.
- mTOR inhibitors such as sirolimus have been shown in vitro to inhibit production of certain growth factors that may affect angiogenesis, fibroblast proliferation, and vascular permeability. Lymphocele, a known surgical complication of renal transplantation, occurred significantly more often in a dose-related fashion in patients treated with sirolimus.
- Appropriate measures should be considered to minimize such complications. Patients with a body mass index (BMI) greater than 30 kg/m2 may be at increased risk of abnormal wound healing based on data from the medical literature.
- There have also been reports of fluid accumulation, including peripheral edema, lymphedema, pleural effusion and pericardial effusions (including hemodynamically significant effusions and tamponade requiring intervention in children and adults), in patients receiving sirolimus.
Hyperlipidemia
- Increased serum cholesterol and triglycerides requiring treatment occurred more frequently in patients treated with sirolimus compared with azathioprine or placebo controls in Studies 1 and 2.
- There were increased incidences of hypercholesterolemia (43-46%) and/or hypertriglyceridemia (45-57%) in patients receiving sirolimus compared with placebo controls (each 23%). The risk/benefit should be carefully considered in patients with established hyperlipidemia before initiating an immunosuppressive regimen including sirolimus.
- Any patient who is administered sirolimus should be monitored for hyperlipidemia. If detected, interventions such as diet, exercise, and lipid-lowering agents should be initiated as outlined by the National Cholesterol Education Program guidelines.
- In clinical trials, the concomitant administration of sirolimus and HMG-CoA reductase inhibitors and/or fibrates appeared to be well-tolerated.
- During sirolimus therapy with cyclosporine, patients administered an HMG-CoA reductase inhibitor and/or fibrate should be monitored for the possible development of rhabdomyolysis and other adverse effects, as described in the respective labeling for these agents.
Renal Function
- Renal function should be closely monitored during the co-administration of sirolimus with cyclosporine, because long-term administration of the combination has been associated with deterioration of renal function. Patients treated with cyclosporine and sirolimus were noted to have higher serum creatinine levels and lower glomerular filtration rates compared with patients treated with cyclosporine and placebo or azathioprine controls (Studies 1 and 2). The rate of decline in renal function in these studies was greater in patients receiving sirolimus and cyclosporine compared with control therapies.
- Appropriate adjustment of the immunosuppressive regimen, including discontinuation of sirolimus and/or cyclosporine, should be considered in patients with elevated or increasing serum creatinine levels. In patients at low- to moderate-immunologic risk, continuation of combination therapy with cyclosporine beyond 4 months following transplantation should only be considered when the benefits outweigh the risks of this combination for the individual patients. Caution should be exercised when using agents (e.g., aminoglycosides and amphotericin B) that are known to have a deleterious effect on renal function.
- In patients with delayed graft function, sirolimus may delay recovery of renal function.
Proteinuria
- Periodic quantitative monitoring of urinary protein excretion is recommended. In a study evaluating conversion from calcineurin inhibitors (CNI) to sirolimus in maintenance renal transplant patients 6-120 months post-transplant, increased urinary protein excretion was commonly observed from 6 through 24 months after conversion to sirolimus compared with CNI continuation.
- Patients with the greatest amount of urinary protein excretion prior to sirolimus conversion were those whose protein excretion increased the most after conversion.New onset nephrosis (nephrotic syndrome) was also reported as a treatment-emergent adverse event in 2.2% of the sirolimus conversion group patients in comparison to 0.4% in the CNI continuation group of patients. Nephrotic range proteinuria (defined as urinary protein to creatinine ratio > 3.5) was also reported in 9.2% in the sirolimus conversion group of patients in comparison to 3.7% in the CNI continuation group of patients. In some patients, reduction in the degree of urinary protein excretion was observed for individual patients following discontinuation of sirolimus.
- The safety and efficacy of conversion from calcineurin inhibitors to sirolimus in maintenance renal transplant patients have not been established.
Interstitial Lung Disease
- Cases of interstitial lung disease (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving immunosuppressive regimens including sirolimus. In some cases, the interstitial lung disease has resolved upon discontinuation or dose reduction of sirolimus. The risk may be increased as the trough sirolimus concentration increases.
De Novo Use Without Cyclosporine
- The safety and efficacy of de novo use of sirolimus without cyclosporine is not established in renal transplant patients. In a multicenter clinical study, de novo renal transplant patients treated with sirolimus, mycophenolate mofetil (MMF), steroids, and an IL-2 receptor antagonist had significantly higher acute rejection rates and numerically higher death rates compared to patients treated with cyclosporine, MMF, steroids, and IL-2 receptor antagonist. A benefit, in terms of better renal function, was not apparent in the treatment arm with de novo use of sirolimus without cyclosporine. These findings were also observed in a similar treatment group of another clinical trial.
Increased Risk of Calcineurin Inhibitor-Induced Hemolytic Uremic Syndrome/Thrombotic Thrombocytopenic Purpura/Thrombotic Microangiopathy (HUS/TTP/TMA)
- The concomitant use of sirolimus with a calcineurin inhibitor may increase the risk of calcineurin inhibitor-induced hemolytic uremic syndrome/thrombotic thrombocytopenic purpura/thrombotic microangiopathy (HUS/TTP/TMA).
Antimicrobial Prophylaxis
- Cases of Pneumocystis carinii pneumonia have been reported in patients not receiving antimicrobial prophylaxis. Therefore, antimicrobial prophylaxis for Pneumocystis carinii pneumonia should be administered for 1 year following transplantation.
- Cytomegalovirus (CMV) prophylaxis is recommended for 3 months after transplantation, particularly for patients at increased risk for CMV disease.
Assay for Sirolimus Therapeutic Drug Monitoring
- The label-recommended 24-hour trough concentration ranges for sirolimus are based on chromatographic methods. Currently in clinical practice, sirolimus whole blood concentrations are being measured by both chromatographic and immunoassay methodologies. These concentration values are not interchangeable.
Skin Cancer Events
- Patients on immunosuppressive therapy are at increased risk for skin cancer. Exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.
Interaction with Strong Inhibitors and Inducers of CYP3A4 and/or P-gp
- Co-administration of sirolimus with strong inhibitors of CYP3A4 and/or P-gp (such as ketoconazole, voriconazole, itraconazole, erythromycin, telithromycin, or clarithromycin) or strong inducers of CYP3A4 and/or P-gp (such as rifampin or rifabutin) is not recommended
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the label.
- Increased susceptibility to infection, lymphoma, and malignancy
- Excess mortality, graft loss, and hepatic artery thrombosis in liver transplant patients.
- Bronchial anastomotic dehiscence in lung transplant patients.
- Hypersensitivity reactions
- Exfoliative dermatitis
- Angioedema
- Fluid Accumulation and Wound Healing.
- Hypertriglyceridemia, hypercholesterolemia
- Decline in renal function in long-term combination of cyclosporine with sirolimus
- Proteinuria
- Interstitial lung disease
- Increased risk of calcineurin inhibitor-induced hemolytic uremic syndrome/thrombotic thrombocytopenic purpura/thrombotic microangiopathy (HUS/TTP/TMA).
- The most common (≥ 30%) adverse reactions observed with sirolimus in clinical studies are: peripheral edema, hypertriglyceridemia, hypertension, hypercholesterolemia, creatinine increased, constipation, abdominal pain, diarrhea, headache, fever, urinary tract infection, anemia, nausea, arthralgia, pain, and thrombocytopenia.
- The following adverse reactions resulted in a rate of discontinuation of > 5% in clinical trials: creatinine increased, hypertriglyceridemia, and thrombotic thrombocytopenic purpura (TTP).
Clinical Studies Experience in Prophylaxis of Organ Rejection Following Renal Transplantation
- The safety and efficacy of sirolimus Oral Solution for the prevention of organ rejection following renal transplantation were assessed in two randomized, double-blind, multicenter, controlled trials. The safety profiles in the two studies were similar.
- The incidence of adverse reactions in the randomized, double-blind, multicenter, placebo-controlled trial (Study 2) in which 219 renal transplant patients received sirolimus Oral Solution 2 mg/day, 208 received sirolimus Oral Solution 5 mg/day, and 124 received placebo is presented in the TABLE below. The study population had a mean age of 46 years (range 15 to 71 years), the distribution was 67% male, and the composition by race was: White (78%), Black (11%), Asian (3%), Hispanic (2%), and Other (5%). All patients were treated with cyclosporine and corticosteroids. Data (≥ 12 months post-transplant) presented in the following TABLE show the adverse reactions that occurred in at least one of the sirolimus treatment groups with an incidence of ≥ 20%.
- The safety profile of the tablet did not differ from that of the oral solution formulation.
- In general, adverse reactions related to the administration of sirolimus were dependent on dose/concentration. Although a daily maintenance dose of 5 mg, with a loading dose of 15 mg, was shown to be safe and effective, no efficacy advantage over the 2 mg dose could be established for renal transplant patients.
- Patients receiving 2 mg of sirolimus Oral Solution per day demonstrated an overall better safety profile than did patients receiving 5 mg of sirolimus Oral Solution per day.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice.
- The following adverse reactions were reported less frequently (≥ 3%, but < 20%)
Body as a Whole
- Sepsis, lymphocele, herpes zoster, herpes simplex.
Cardiovascular
- Venous thromboembolism (including pulmonary embolism, deep venous thrombosis), tachycardia.
Digestive System
- Stomatitis.
Hematologic and Lymphatic System
- Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), leukopenia.
Metabolic/Nutritional
- Abnormal healing, increased lactic dehydrogenase (LDH), hypokalemia.
Musculoskeletal System
- Bone necrosis.
Respiratory System
- Pneumonia, epistaxis.
Skin
- Melanoma, squamous cell carcinoma, basal cell carcinoma.
Urogenital System
- Pyelonephritis, decline in renal function (creatinine increased) in long-term combination of cyclosporine with sirolimus.
- Less frequently (< 3%) occurring adverse reactions included: lymphoma/post-transplant lymphoproliferative disorder, mycobacterial infections (including M. tuberculosis), pancreatitis, cytomegalovirus (CMV), and Epstein-Barr virus.
Increased Serum Cholesterol and Triglycerides
- The use of sirolimus in renal transplant patients was associated with increased serum cholesterol and triglycerides that may require treatment.
- In Studies 1 and 2, in de novo renal transplant patients who began the study with fasting, total serum cholesterol< 200 mg/dL or fasting, total serum triglycerides < 200 mg/dL, there was an increased incidence of hypercholesterolemia (fasting serum cholesterol > 240 mg/dL) or hypertriglyceridemia (fasting serum triglycerides > 500 mg/dL), respectively, in patients receiving both sirolimus 2 mg and sirolimus 5 mg compared with azathioprine and placebo controls.
- Treatment of new-onset hypercholesterolemia with lipid-lowering agents was required in 42‑52% of patients enrolled in the sirolimus arms of Studies 1 and 2 compared with 16% of patients in the placebo arm and 22% of patients in the azathioprine arm.
Abnormal Healing
- Abnormal healing events following transplant surgery include fascial dehiscence, incisional hernia, and anastomosis disruption (e.g., wound, vascular, airway, ureteral, biliary).
Malignancies
- The TABLE below summarizes the incidence of malignancies in the two controlled trials (Studies 1 and 2) for the prevention of acute rejection.
- At 24 months (Study 1) and 36 months (Study 2), there were no significant differences among treatment groups.
Sirolimus Following Cyclosporine Withdrawal
- The incidence of adverse reactions was determined through 36 months in a randomized, multicenter, controlled trial (Study 3) in which 215 renal transplant patients received sirolimus as a maintenance regimen following cyclosporine withdrawal, and 215 patients received sirolimus with cyclosporine therapy.
- All patients were treated with corticosteroids. The safety profile prior to randomization (start of cyclosporine withdrawal) was similar to that of the 2 mg sirolimus groups in Studies 1 and 2.
- Following randomization (at 3 months), patients who had cyclosporine eliminated from their therapy experienced higher incidences of the following adverse reactions: abnormal liver function tests (including increased AST/SGOT and increased ALT/SGPT), hypokalemia, thrombocytopenia, and abnormal healing. Conversely, the incidence of the following adverse events was higher in patients who remained on cyclosporine than those who had cyclosporine withdrawn from therapy: hypertension, cyclosporine toxicity, increased creatinine, abnormal kidney function, toxic nephropathy, edema, hyperkalemia, hyperuricemia, and gum hyperplasia. Mean systolic and diastolic blood pressure improved significantly following cyclosporine withdrawal.
Malignancies
- The incidence of malignancies in Study 3 [see Clinical Studies (14.2)] is presented in the TABLE following.
- In Study 3, the incidence of lymphoma/lymphoproliferative disease was similar in all treatment groups. The overall incidence of malignancy was higher in patients receiving sirolimus plus cyclosporine compared with patients who had cyclosporine withdrawn. Conclusions regarding these differences in the incidence of malignancy could not be made because Study 3 was not designed to consider malignancy risk factors or systematically screen subjects for malignancy. In addition, more patients in the sirolimus with cyclosporine group had a pretransplantation history of skin carcinoma.
High-Immunologic Risk Patients
- Safety was assessed in 224 patients who received at least one dose of sirolimus with cyclosporine [see Clinical Studies (14.3)]. Overall, the incidence and nature of adverse events was similar to those seen in previous combination studies with sirolimus. The incidence of malignancy was 1.3% at 12 months.
Conversion from Calcineurin Inhibitors to sirolimus in Maintenance Renal Transplant Population
- The safety and efficacy of conversion from calcineurin inhibitors to sirolimus in maintenance renal transplant population have not been established [see Clinical Studies (14.4)]. In an ongoing study evaluating the safety and efficacy of conversion from calcineurin inhibitors to sirolimus (initial target sirolimus concentrations of 12-20 ng/mL, and then 8-20 ng/mL, by chromatographic assay) in maintenance renal transplant patients, enrollment was stopped in the subset of patients (n = 87) with a baseline glomerular filtration rate of less than 40 mL/min. There was a higher rate of serious adverse events, including pneumonia, acute rejection, graft loss and death, in this stratum of the sirolimus treatment arm.
- The subset of patients with a baseline glomerular filtration rate of less than 40 mL/min had 2 years of follow-up after randomization. In this population, the rate of pneumonia was 15/58 vs. 4/29, graft loss (excluding death with functioning graft loss) was 13/58 vs. 9/29, and death was 9/58 vs. 1/29 in the sirolimus conversion group and CNI continuation group, respectively.
- In the subset of patients with a baseline glomerular filtration rate of greater than 40 mL/min, there was no benefit associated with conversion with regard to improvement in renal function and a greater incidence of proteinuria in the sirolimus conversion arm.
- Overall in this study, a 5-fold increase in the reports of tuberculosis among sirolimus (11/551) and comparator (1/273) treatment groups was observed with 2:1 randomization scheme.
Pediatrics
- Safety was assessed in a controlled clinical trial in pediatric (< 18 years of age) renal transplant patients considered at high-immunologic risk, defined as a history of one or more acute allograft rejection episodes and/or the presence of chronic allograft nephropathy on a renal biopsy.
- The use of sirolimus in combination with calcineurin inhibitors and corticosteroids was associated with a higher incidence of deterioration of renal function (creatinine increased) compared to calcineurin inhibitor-based therapy, serum lipid abnormalities (including, but not limited to, increased serum triglycerides and cholesterol), and urinary tract infections.
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of sirolimus. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole – Lymphedema
- Cardiovascular – Pericardial effusion (including hemodynamically significant effusions and tamponade requiring intervention in children and adults).
- Hematological/Lymphatic – The concomitant use of sirolimus with a calcineurin inhibitor may increase the risk of calcineurin inhibitor-induced HUS/TTP/TMA; pancytopenia, neutropenia.
Hepatobiliary Disorders
- Hepatotoxicity, including fatal hepatic necrosis, with elevated sirolimus trough concentrations.
Immune System
- Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, angioedema, and hypersensitivity vasculitis.
Infections
- Tuberculosis.
Metabolic/Nutritional
- Liver function test abnormal, AST/SGOT increased, ALT/SGPT increased, hypophosphatemia, hyperglycemia.
Respiratory
- Cases of interstitial lung disease (including pneumonitis, bronchiolitis obliterans organizing pneumonia [BOOP], and pulmonary fibrosis), some fatal, with no identified infectious etiology have occurred in patients receiving immunosuppressive regimens including sirolimus. In some cases, the interstitial lung disease has resolved upon discontinuation or dose reduction of sirolimus. The risk may be increased as the sirolimus trough concentration increases; pulmonary hemorrhage; pleural effusion; alveolar proteinosis
Skin
- Exfoliative dermatitis.
Urogenital
- Nephrotic syndrome, proteinuria, focal segmental glomerulosclerosis. Azoospermia has been reported with the use of sirolimus and has been reversible upon discontinuation of sirolimus in most cases.
Drug Interactions
- Sirolimus is known to be a substrate for both cytochrome P‑450 3A4 (CYP3A4) and p‑glycoprotein (P‑gp). Inducers of CYP3A4 and P‑gp may decrease sirolimus concentrations whereas inhibitors of CYP3A4 and P‑gp may increase sirolimus concentrations.
Use with Cyclosporine
- Cyclosporine, a substrate and inhibitor of CYP3A4 and P‑gp, was demonstrated to increase sirolimus concentrations when co‑administered with sirolimus. In order to diminish the effect of this interaction with cyclosporine, it is recommended that sirolimus be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and/or cyclosporine capsules (MODIFIED).
- If cyclosporine is withdrawn from combination therapy with sirolimus, higher doses of sirolimus are needed to maintain the recommended sirolimus trough concentration ranges.
- Strong Inducers and Strong Inhibitors of CYP3A4 and P-gp
Avoid concomitant use of sirolimus with strong inducers (e.g., rifampin, rifabutin) and strong inhibitors (e.g., ketoconazole, voriconazole, itraconazole, erythromycin, telithromycin, clarithromycin) of CYP3A4 and P-gp. Alternative agents with lesser interaction potential with sirolimus should be considered.
Grapefruit Juice
- Because grapefruit juice inhibits the CYP3A4-mediated metabolism of sirolimus, it must not be taken with or be used for dilution of sirolimus.
Inducers or Inhibitors of CYP3A4 and P-gp
- Exercise caution when using sirolimus with drugs or agents that are modulators of CYP3A4 and P-gp. The dosage of sirolimus and/or the co-administered drug may need to be adjusted.
- Drugs that could increase sirolimus blood concentrations:
Bromocriptione, cimetidine, cisapride, clotrimazole, danazol, diltiazem, fluconazole, HIV‑protease inhibitors (e.g., ritonavir, indinavir), metoclopramide, nicardipine, troleandomycin, verapamil Drugs and other agents that could decrease sirolimus concentrations:
- Carbamazepine, phenobarbital, phenytoin, rifapentine, St. John's Wort (Hypericum perforatum)
- Drugs with concentrations that could increase when given with sirolimus:
- Verapamil
Vaccination
- Immunosuppressants may affect response to vaccination. Therefore, during treatment with sirolimus, vaccination may be less effective. The use of live vaccines should be avoided; live vaccines may include, but are not limited to, the following: measles, mumps, rubella, oral polio, BCG, yellow fever, varicella, and TY21a typhoid.
Use in Specific Populations
Pregnancy
- Sirolimus was embryo/fetotoxic in rats when given in doses approximately 0.2 to 0.5 the human doses (adjusted for body surface area). Embryo/fetotoxicity was manifested as mortality and reduced fetal weights (with associated delays in skeletal ossification). However, no teratogenesis was evident. In combination with cyclosporine, rats had increased embryo/feto mortality compared with sirolimus alone. There were no effects on rabbit development at a maternally toxic dosage approximately 0.3 to 0.8 times the human doses (adjusted for body surface area). There are no adequate and well‑controlled studies in pregnant women. Effective contraception must be initiated before sirolimus therapy, during sirolimus therapy, and for 12 weeks after sirolimus therapy has been stopped. Sirolimus should be used during pregnancy only if the potential benefit outweighs the potential risk to the embryo/fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sirolimus in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sirolimus during labor and delivery.
Nursing Mothers
- Sirolimus is excreted in trace amounts in milk of lactating rats. It is not known whether sirolimus is excreted in human milk. The pharmacokinetic and safety profiles of sirolimus in infants are not known. Because many drugs are excreted in human milk, and because of the potential for adverse reactions in nursing infants from sirolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and efficacy of sirolimus in pediatric patients < 13 years have not been established.
- The safety and efficacy of sirolimus Oral Solution and sirolimus Tablets have been established in children ≥ 13 years judged to be at low- to moderate-immunologic risk. Use of sirolimus Oral Solution and sirolimus Tablets in this subpopulation of children ≥ 13 years is supported by evidence from adequate and well-controlled trials of sirolimus Oral Solution in adults with additional pharmacokinetic data in pediatric renal transplantation patients.
- Safety and efficacy information from a controlled clinical trial in pediatric and adolescent (< 18 years of age) renal transplant patients judged to be at high-immunologic risk, defined as a history of one or more acute rejection episodes and/or the presence of chronic allograft nephropathy, do not support the chronic use of sirolimus Oral Solution or Tablets in combination with calcineurin inhibitors and corticosteroids, due to the higher incidence of lipid abnormalities and deterioration of renal function associated with these immunosuppressive regimens compared to calcineurin inhibitors, without increased benefit with respect to acute rejection, graft survival, or patient survival.
Geriatic Use
- Clinical studies of sirolimus Oral Solution or Tablets did not include sufficient numbers of patients ≥ 65 years to determine whether they respond differently from younger patients. Data pertaining to sirolimus trough concentrations suggest that dose adjustments based upon age in geriatric renal patients are not necessary. Differences in responses between the elderly and younger patients have not been identified. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Sirolimus with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sirolimus with respect to specific racial populations.
Renal Impairment
- Dosage adjustment is not required in patients with renal impairment .
Hepatic Impairment
- The maintenance dose of sirolimus should be reduced in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sirolimus in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sirolimus in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the sirolimus dosage form is made, and during concurrent administration of strong CYP3A4 inducers and inhibitors.
- Therapeutic drug monitoring should not be the sole basis for adjusting sirolimus therapy. Careful attention should be made to clinical signs/symptoms, tissue biopsy findings, and laboratory parameters.
- When used in combination with cyclosporine, sirolimus trough concentrations should be maintained within the target-range.
- Following cyclosporine withdrawal in transplant patients at low- to moderate-immunologic risk, the target sirolimus trough concentrations should be 16 to 24 ng/mL for the first year following transplantation. Thereafter, the target sirolimus concentrations should be 12 to 20 ng/mL.
- The above recommended 24-hour trough concentration ranges for sirolimus are based on chromatographic methods. On average, chromatographic methods (HPLC UV or LC/MS/MS) yield results that are approximately 20% lower than the immunoassay for whole blood concentration determinations. Currently in clinical practice, sirolimus whole blood concentrations are being measured by both chromatographic and immunoassay methodologies. Because the measured sirolimus whole blood concentrations depend on the type of assay used, the concentrations obtained by these different methodologies are not interchangeable.
- Adjustments to the targeted range should be made according to the assay utilized to determine sirolimus trough concentrations.
- Therapeutic drug monitoring should be used to maintain sirolimus drug levels within the target-range.
- Therapeutic drug monitoring is necessary in all patients with hepatic impairment.
- Renal function should be monitored, and appropriate adjustment of the immunosuppressive regimen should be considered in patients with elevated or increasing serum creatinine levels.
- Periodic quantitative monitoring of urinary protein excretion is recommended.
- During sirolimus therapy with cyclosporine, patients administered an HMG-CoA reductase inhibitor and/or fibrate should be monitored for the possible development of rhabdomyolysis and other adverse effects, as described in the respective labeling for these agents.
- Any patient who is administered sirolimus should be monitored for hyperlipidemia.
- Monitoring of sirolimus trough concentrations is recommended for all patients, especially in those patients likely to have altered drug metabolism, in patients≥ 13 years who weigh less than 40 kg, in patients with hepatic impairment,when a change in the sirolimus dosage form is made, and during concurrent administration of strong CYP3A4 inducers and inhibitors.
- Once sirolimus maintenance dose is adjusted, patients should continue on the new maintenance dose for at least 7 to 14 days before further dosage adjustment with concentration monitoring.
IV Compatibility
There is limited information regarding IV Compatibility of Sirolimus in the drug label.
Overdosage
- Reports of overdose with sirolimus have been received; however, experience has been limited. In general, the adverse effects of overdose are consistent with those listed in the adverse reactions section.
- General supportive measures should be followed in all cases of overdose. Based on the low aqueous solubility and high erythrocyte and plasma protein binding of sirolimus, it is anticipated that sirolimus is not dialyzable to any significant extent. In mice and rats, the acute oral LD50 was greater than 800 mg/kg.
Pharmacology
Mechanism of Action
- Sirolimus inhibits T-lymphocyte activation and proliferation that occurs in response to antigenic and cytokine (Interleukin [IL]-2, IL-4, and IL-15) stimulation by a mechanism that is distinct from that of other immunosuppressants. Sirolimus also inhibits antibody production. In cells, sirolimus binds to the immunophilin, FK Binding Protein-12 (FKBP-12), to generate an immunosuppressive complex. The sirolimus:FKBP-12 complex has no effect on calcineurin activity. This complex binds to and inhibits the activation of the mammalian Target Of Rapamycin (mTOR), a key regulatory kinase. This inhibition suppresses cytokine-driven T-cell proliferation, inhibiting the progression from the G1 to the S phase of the cell cycle.
- Studies in experimental models show that sirolimus prolongs allograft (kidney, heart, skin, islet, small bowel, pancreatico-duodenal, and bone marrow) survival in mice, rats, pigs, and/or primates. Sirolimus reverses acute rejection of heart and kidney allografts in rats and prolongs the graft survival in presensitized rats. In some studies, the immunosuppressive effect of sirolimus lasts up to 6 months after discontinuation of therapy. This tolerization effect is alloantigen-specific.
- In rodent models of autoimmune disease, sirolimus suppresses immune-mediated events associated with systemic lupus erythematosus, collagen-induced arthritis, autoimmune type I diabetes, autoimmune myocarditis, experimental allergic encephalomyelitis, graft-versus-host disease, and autoimmune uveoretinitis.
Structure
- Sirolimus is an immunosuppressive agent. Sirolimus is a macrocyclic lactone produced by Streptomyces hygroscopicus. The chemical name of sirolimus (also known as rapamycin) is (3S,6R,7E,9R,10R,12R,14S,15E,17E,19E,21S,23S,26R,27R,34aS)-9,10,12,13,14,21,22,23,24,25,26,27,32,33,34, 34a-hexadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-1-methylethyl]-10,21-dimethoxy-6,8,12,14,20,26-hexamethyl-23,27-epoxy-3H-pyrido[2,1-c][1,4] oxaazacyclohentriacontine-1,5,11,28,29 (4H,6H,31H)-pentone. Its molecular formula is C51H79NO13 and its molecular weight is 914.2. The structural formula of sirolimus is illustrated as follows.
- Sirolimus is a white to off-white powder and is insoluble in water, but freely soluble in benzyl alcohol, chloroform, acetone, and acetonitrile.
- Sirolimus is available for administration as an oral solution containing 1 mg/mL sirolimus. Sirolimus is also available as a white, triangular-shaped tablet containing 1 mg sirolimus, and as a yellow-to-beige triangular-shaped tablet containing 2 mg sirolimus.
- The inactive ingredients in sirolimus Oral Solution are Phosal 50 PG® (phosphatidylcholine, propylene glycol, mono- and di-glycerides, ethanol, soy fatty acids, and ascorbyl palmitate) and polysorbate 80. Sirolimus Oral Solution contains 1.5% - 2.5% ethanol.
- The inactive ingredients in sirolimus Tablets include sucrose, lactose, polyethylene glycol 8000, calcium sulfate, microcrystalline cellulose, pharmaceutical glaze, talc, titanium dioxide, magnesium stearate, povidone, poloxamer 188, polyethylene glycol 20,000, glyceryl monooleate, carnauba wax, dl-alpha tocopherol, and other ingredients. The 2 mg dosage strength also contains iron oxide yellow 10 and iron oxide brown 70.
Pharmacodynamics
- Orally-administered sirolimus, at doses of 2 mg/day and 5 mg/day, significantly reduced the incidence of organ rejection in low- to moderate-immunologic risk renal transplant patients at 6 months following transplantation compared with either azathioprine or placebo. There was no demonstrable efficacy advantage of a daily maintenance dose of 5 mg with a loading dose of 15 mg over a daily maintenance dose of 2 mg with a loading dose of 6 mg. Therapeutic drug monitoring should be used to maintain sirolimus drug levels within the target-range.
Pharmacokinetics
- Sirolimus pharmacokinetics activity have been determined following oral administration in healthy subjects, pediatric patients, hepatically impaired patients, and renal transplant patients.
- The pharmacokinetic parameters of sirolimus in low- to moderate-immunologic risk adult renal transplant patients following multiple dosing with sirolimus 2 mg daily, in combination with cyclosporine and corticosteroids, is summarized in the following TABLE.
- Whole blood trough sirolimus concentrations, as measured by LC/MS/MS in renal transplant patients, were significantly correlated with AUCτ,ss. Upon repeated, twice-daily administration without an initial loading dose in a multiple-dose study, the average trough concentration of sirolimus increases approximately 2- to 3-fold over the initial 6 days of therapy, at which time steady-state is reached. A loading dose of 3 times the maintenance dose will provide near steady-state concentrations within 1 day in most patients.
Absorption
- Following administration of sirolimus Oral Solution, the mean times to peak concentration (tmax) of sirolimus are approximately 1 hour and 2 hours in healthy subjects and renal transplant patients, respectively. The systemic availability of sirolimus is low, and was estimated to be approximately 14% after the administration of sirolimus Oral Solution. In healthy subjects, the mean bioavailability of sirolimus after administration of the tablet is approximately 27% higher relative to the solution.
- Sirolimus tablets are not bioequivalent to the solution; however, clinical equivalence has been demonstrated at the 2 mg dose level. Sirolimus concentrations, following the administration of sirolimus Oral Solution to stable renal transplant patients, are dose-proportional between 3 and 12 mg/m2.
Food Effects
- To minimize variability in sirolimus concentrations, both sirolimus Oral Solution and Tablets should be taken consistently with or without food. In healthy subjects, a high-fat meal (861.8 kcal, 54.9% kcal from fat) increased the mean total exposure (AUC) of sirolimus by 23 to 35%, compared with fasting. The effect of food on the mean sirolimus Cmax was inconsistent depending on the sirolimus dosage form evaluated.
Distribution
- The mean (± SD) blood-to-plasma ratio of sirolimus was 36 ± 18 in stable renal allograft patients, indicating that sirolimus is extensively partitioned into formed blood elements. The mean volume of distribution (Vss/F) of sirolimus is 12 ± 8 L/kg. Sirolimus is extensively bound (approximately 92%) to human plasma proteins, mainly serum albumin (97%), α1-acid glycoprotein, and lipoproteins.
Metabolism
- Sirolimus is a substrate for both CYP3A4 and P-gp. Sirolimus is extensively metabolized in the intestinal wall and liver and undergoes counter-transport from enterocytes of the small intestine into the gut lumen. Inhibitors of CYP3A4 and P-gp increase sirolimus concentrations. Inducers of CYP3A4 and P-gp decrease sirolimus concentrations.
- Sirolimus is extensively metabolized by O‑demethylation and/or hydroxylation. Seven (7) major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, fecal, and urine samples. Sirolimus is the major component in human whole blood and contributes to more than 90% of the immunosuppressive activity.
Excretion
- After a single dose of [14C] sirolimus oral solution in healthy volunteers, the majority (91%) of radioactivity was recovered from the feces, and only a minor amount (2.2%) was excreted in urine. The mean ± SD terminal elimination half life (t½) of sirolimus after multiple dosing in stable renal transplant patients was estimated to be about 62 ± 16 hours.
- Sirolimus Concentrations (Chromatographic Equivalent) Observed in Phase 3 Clinical Studies
- The FOLLOWING sirolimus concentrations (chromatographic equivalent) were observed in phase 3 clinical studies.
- The withdrawal of cyclosporine and concurrent increases in sirolimus trough concentrations to steady-state required approximately 6 weeks. Following cyclosporine withdrawal, larger sirolimus doses were required due to the absence of the inhibition of sirolimus metabolism and transport by cyclosporine and to achieve higher target sirolimus trough concentrations during concentration-controlled administration.
Pharmacokinetics in Specific Populations
Hepatic Impairment=
- Sirolimus was administered as a single, oral dose to subjects with normal hepatic function and to patients with Child-Pugh classification A (mild), B (moderate), or C (severe) hepatic impairment. Compared with the values in the normal hepatic function group, the patients with mild, moderate, and severe hepatic impairment had 43%, 94%, and 189% higher mean values for sirolimus AUC, respectively, with no statistically significant differences in mean Cmax. As the severity of hepatic impairment increased, there were steady increases in mean sirolimus t1/2, and decreases in the mean sirolimus clearance normalized for body weight (CL/F/kg).
- The maintenance dose of sirolimus should be reduced by approximately one third in patients with mild‑to‑moderate hepatic impairment and by approximately one half in patients with severe hepatic impairment.
- It is not necessary to modify the sirolimus loading dose in patients with mild, moderate, and severe hepatic impairment. Therapeutic drug monitoring is necessary in all patients with hepatic impairment.
Renal Impairment
- The effect of renal impairment on the pharmacokinetics of sirolimus is not known. However, there is minimal (2.2%) renal excretion of the drug or its metabolites in healthy volunteers. The loading and the maintenance doses of sirolimus need not be adjusted in patients with renal impairment.
Pediatric
- Sirolimus pharmacokinetic data were collected in concentration-controlled trials of pediatric renal transplant patients who were also receiving cyclosporine and corticosteroids. The target ranges for trough concentrations were either 10-20 ng/mL for the 21 children receiving tablets, or 5-15 ng/mL for the one child receiving oral solution. The children aged 6-11 years (n = 8) received mean ± SD doses of 1.75 ± 0.71 mg/day (0.064 ± 0.018 mg/kg, 1.65 ± 0.43 mg/m2). The children aged 12-18 years (n = 14) received mean ± SD doses of 2.79 ± 1.25 mg/day (0.053 ± 0.0150 mg/kg, 1.86± 0.61 mg/m2). At the time of sirolimus blood sampling for pharmacokinetic evaluation, the majority (80%) of these pediatric patients received the sirolimus dose at 16 hours after the once-daily cyclosporine dose.
Geriatric
- Clinical studies of sirolimus did not include a sufficient number of patients > 65 years of age to determine whether they will respond differently than younger patients. After the administration of sirolimus Oral Solution or Tablets, sirolimus trough concentration data in renal transplant patients > 65 years of age were similar to those in the adult population 18 to 65 years of age.
Gender
- Sirolimus clearance in males was 12% lower than that in females; male subjects had a significantly longer t1/2 than did female subjects (72.3 hours versus 61.3 hours). Dose adjustments based on gender are not recommended.
Race
- In the phase 3 trials using sirolimus solution or tablets and cyclosporine oral solution [MODIFIED] (e.g., Neoral® Oral Solution) and/or cyclosporine capsules [MODIFIED] (e.g., Neoral® Soft Gelatin Capsules) [see Clinical Studies (14)], there were no significant differences in mean trough sirolimus concentrations over time between Black (n = 190) and non-Black (n = 852) patients during the first 6 months after transplantation.
Drug-Drug Interactions
- Sirolimus is known to be a substrate for both cytochrome CYP3A4 and P-gp. The pharmacokinetic interaction between sirolimus and concomitantly administered drugs is discussed below. Drug interaction studies have not been conducted with drugs other than those described below.
Cyclosporine
- Cyclosporine is a substrate and inhibitor of CYP3A4 and P-gp. Sirolimus should be taken 4 hours after administration of cyclosporine oral solution (MODIFIED) and/or cyclosporine capsules (MODIFIED). Sirolimus concentrations may decrease when cyclosporine is discontinued, unless the sirolimus dose is increased.
- In a single-dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg sirolimus Tablets either simultaneously or 4 hours after a 300-mg dose of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). For simultaneous administration, mean Cmax and AUC were increased by 512% and 148%, respectively, relative to administration of sirolimus alone. However, when given 4 hours after cyclosporine administration, sirolimus Cmax and AUC were both increased by only 33% compared with administration of sirolimus alone.
- In a single dose drug-drug interaction study, 24 healthy volunteers were administered 10 mg sirolimus Oral Solution either simultaneously or 4 hours after a 300 mg dose of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). For simultaneous administration, the mean Cmax and AUC of sirolimus, following simultaneous administration were increased by 116% and 230%, respectively, relative to administration of sirolimus alone. However, when given 4 hours after Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) administration, sirolimus Cmax and AUC were increased by only 37% and 80%, respectively, compared with administration of sirolimus alone.
- In a single-dose cross-over drug-drug interaction study, 33 healthy volunteers received 5 mg sirolimus Oral Solution alone, 2 hours before, and 2 hours after a 300 mg dose of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). When given 2 hours before Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) administration, sirolimus Cmax and AUC were comparable to those with administration of sirolimus alone. However, when given 2 hours after, the mean Cmax and AUC of sirolimus were increased by 126% and 141%, respectively, relative to administration of sirolimus alone.
- Mean cyclosporine Cmax and AUC were not significantly affected when sirolimus Oral Solution was given simultaneously or when administered 4 hours after Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]). However, after multiple-dose administration of sirolimus given 4 hours after Neoral® in renal post-transplant patients over 6 months, cyclosporine oral-dose clearance was reduced, and lower doses of Neoral® Soft Gelatin Capsules (cyclosporine capsules [MODIFIED]) were needed to maintain target cyclosporine concentration.
- In a multiple-dose study in 150 psoriasis patients, sirolimus 0.5, 1.5, and 3 mg/m2/day was administered simultaneously with Sandimmune® Oral Solution (cyclosporine Oral Solution) 1.25 mg/kg/day. The increase in average sirolimus trough concentrations ranged between 67% to 86% relative to when sirolimus was administered without cyclosporine. The intersubject variability (% CV) for sirolimus trough concentrations ranged from 39.7% to 68.7%. There was no significant effect of multiple-dose sirolimus on cyclosporine trough concentrations following Sandimmune® Oral Solution (cyclosporine oral solution) administration. However, the % CV was higher (range 85.9% - 165%) than those from previous studies.
Diltiazem
- Diltiazem is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 10 mg of sirolimus oral solution and 120 mg of diltiazem to 18 healthy volunteers significantly affected the bioavailability of sirolimus. Sirolimus Cmax, tmax, and AUC were increased 1.4-, 1.3-, and 1.6-fold, respectively. Sirolimus did not affect the pharmacokinetics of either diltiazem or its metabolites desacetyldiltiazem and desmethyldiltiazem.
Erythromycin
- Erythromycin is a substrate and inhibitor of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and erythromycin is not recommended. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 800 mg q 8h of erythromycin as erythromycin ethylsuccinate tablets at steady state to 24 healthy volunteers significantly affected the bioavailability of sirolimus and erythromycin. Sirolimus Cmax and AUC were increased 4.4- and 4.2-fold respectively and tmax was increased by 0.4 hr. Erythromycin Cmax and AUC were increased 1.6- and 1.7-fold, respectively, and tmax was increased by 0.3 hr.
Ketoconazole
- Ketoconazole is a strong inhibitor of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and ketoconazole is not recommended. Multiple-dose ketoconazole administration significantly affected the rate and extent of absorption and sirolimus exposure after administration of sirolimus Oral Solution, as reflected by increases in sirolimus Cmax, tmax, and AUC of 4.3-fold, 38%, and 10.9-fold, respectively. However, the terminal t½ of sirolimus was not changed. Single-dose sirolimus did not affect steady-state 12-hour plasma ketoconazole concentrations.
Rifampin
- Rifampin is a strong inducer of CYP3A4 and P-gp; co-administration of sirolimus oral solution or tablets and rifampin is not recommended. In patients where rifampin is indicated, alternative therapeutic agents with less enzyme induction potential should be considered. Pretreatment of 14 healthy volunteers with multiple doses of rifampin, 600 mg daily for 14 days, followed by a single 20-mg dose of sirolimus oral solution, greatly decreased sirolimus AUC and Cmax by about 82% and 71%, respectively.
Verapamil
- Verapamil is a substrate and inhibitor of CYP3A4 and P-gp; sirolimus concentrations should be monitored and a dose adjustment may be necessary. The simultaneous oral administration of 2 mg daily of sirolimus oral solution and 180 mg q 12h of verapamil at steady state to 26 healthy volunteers significantly affected the bioavailability of sirolimus and verapamil. Sirolimus Cmax and AUC were increased 2.3- and 2.2-fold, respectively, without substantial change in tmax. The Cmax and AUC of the pharmacologically active S(-) enantiomer of verapamil were both increased 1.5-fold and tmax was decreased by 1.2 hr.
- Drugs Which May Be Co-administered Without Dose Adjustment
Clinically significant pharmacokinetic drug-drug interactions were not observed in studies of drugs listed below. Sirolimus and these drugs may be co-administered without dose adjustments.
- Acyclovir
- Atorvastatin
- Digoxin
- Glyburide
- Nifedipine
- Norgestrel/ethinyl estradiol (Lo/Ovral®)
- Prednisolone
- Sulfamethoxazole/trimethoprim (Bactrim®)
Other Drug-Drug Interactions
- Co-administration of sirolimus with other known strong inhibitors of CYP3A4 and/or P-gp (such as voriconazole, itraconazole, telithromycin, or clarithromycin) or other known strong inducers of CYP3A4 and/or P-gp (such as rifabutin) is not recommended. In patients in whom strong inhibitors or inducers of CYP3A4 are indicated, alternative therapeutic agents with less potential for inhibition or induction of CYP3A4 should be considered.
- Care should be exercised when drugs or other substances that are substrates and/or inhibitors or inducers of CYP3A4 are administered concomitantly with sirolimus. Other drugs that have the potential to increase sirolimus blood concentrations include (but are not limited to):
- Calcium channel blockers
- Nicardipine.
- Antifungal agent
- Clotrimazole, fluconazole.
- Antibiotics
- Troleandomycin.
- Gastrointestinal prokinetic agents
- Cisapride, metoclopramide.
- Other drugs
- Bromocriptine, cimetidine, danazol, HIV-protease inhibitors (e.g., ritonavir, indinavir).
- Other drugs that have the potential to decrease sirolimus concentrations include (but are not limited to):
- Anticonvulsants
- Carbamazepine, phenobarbital, phenytoin.
- Antibiotics
- Rifapentine.
Other Drug-Food Interactions
- Grapefruit juice reduces CYP3A4-mediated drug metabolism. Grapefruit juice must not be taken with or used for dilution of sirolimus.
Drug-Herb Interactions
- St. John's Wort (hypericum perforatum) induces CYP3A4 and P-gp. Since sirolimus is a substrate for both cytochrome CYP3A4 and P-gp, there is the potential that the use of St. John's Wort in patients receiving sirolimus could result in reduced sirolimus concentrations.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies were conducted in mice and rats. In an 86-week female mouse study at sirolimus doses 30 to 120 times higher than the 2 mg daily clinical dose (adjusted for body surface area), there was a statistically significant increase in malignant lymphoma at all dose levels compared with controls.
- In a second mouse study at dosages that were approximately 3 to 16 times the clinical dose (adjusted for body surface area), hepatocellular adenoma and carcinoma in males were considered sirolimus-related. In the 104-week rat study at dosages equal to or lower than the clinical dose of 2 mg daily (adjusted for body surface area), there were no significant findings.
- Sirolimus was not genotoxic in the in vitro bacterial reverse mutation assay, the Chinese hamster ovary cell chromosomal aberration assay, the mouse lymphoma cell forward mutation assay, or the in vivo mouse micronucleus assay.
- Fertility was diminished slightly in both male and female rats following oral administration of sirolimus at doses approximately 10 times or 2 times, respectively, the clinical dose of 2 mg daily (adjusted for body surface area). In male rats, atrophy of testes, epididymides, prostate, seminiferous tubules and/or reduction in sperm counts were observed. In female rats, reduced size of ovaries and uteri was observed. Reduction of sperm count in male rats was reversible upon cessation of dosing in one study. Testicular tubular degeneration was also seen in a 4‑week intravenous study of sirolimus in monkeys at doses that were approximately equal to the clinical dose (adjusted for body surface area).
Clinical Studies
Prophylaxis of Organ Rejection
Sirolimus Oral Solution
- The safety and efficacy of sirolimus Oral Solution for the prevention of organ rejection following renal transplantation were assessed in two randomized, double-blind, multicenter, controlled trials. These studies compared two dose levels of sirolimus Oral Solution (2 mg and 5 mg, once daily) with azathioprine (Study 1) or placebo (Study 2) when administered in combination with cyclosporine and corticosteroids. Study 1 was conducted in the United States at 38 sites. Seven hundred nineteen (719) patients were enrolled in this trial and randomized following transplantation; 284 were randomized to receive sirolimus Oral Solution 2 mg/day; 274 were randomized to receive sirolimus Oral Solution 5 mg/day, and 161 to receive azathioprine 2-3 mg/kg/day. Study 2 was conducted in Australia, Canada, Europe, and the United States, at a total of 34 sites. Five hundred seventy-six (576) patients were enrolled in this trial and randomized before transplantation; 227 were randomized to receive sirolimus Oral Solution 2 mg/day; 219 were randomized to receive sirolimus Oral Solution 5 mg/day, and 130 to receive placebo. In both studies, the use of antilymphocyte antibody induction therapy was prohibited. In both studies, the primary efficacy endpoint was the rate of efficacy failure in the first 6 months after transplantation. Efficacy failure was defined as the first occurrence of an acute rejection episode (confirmed by biopsy), graft loss, or death.
- The TABLES below summarize the results of the primary efficacy analyses from these trials. Sirolimus Oral Solution, at doses of 2 mg/day and 5 mg/day, significantly reduced the incidence of efficacy failure (statistically significant at the< 0.025 level; nominal significance level adjusted for multiple [2] dose comparisons) at 6 months following transplantation compared with both azathioprine and placebo.
- The reduction in the incidence of first biopsy-confirmed acute rejection episodes in patients treated with sirolimus compared with the control groups included a reduction in all grades of rejection.
- In Study 1, which was prospectively stratified by race within center, efficacy failure was similar for sirolimus Oral Solution 2 mg/day and lower for sirolimus Oral Solution 5 mg/day compared with azathioprine in Black patients. In Study 2, which was not prospectively stratified by race, efficacy failure was similar for both sirolimus Oral Solution doses compared with placebo in Black patients. The decision to use the higher dose of sirolimus Oral Solution in Black patients must be weighed against the increased risk of dose-dependent adverse events that were observed with the sirolimus Oral Solution 5-mg dose.
- Mean glomerular filtration rates (GFR) post-transplant were calculated by using the Nankivell equation at 12 and 24 months for Study 1, and 12 and 36 months for Study 2. Mean GFR was lower in patients treated with cyclosporine and sirolimus Oral Solution compared with those treated with cyclosporine and the respective azathioprine or placebo control.
- Within each treatment group in Studies 1 and 2, mean GFR at one-year post-transplant was lower in patients who experienced at least one episode of biopsy-proven acute rejection, compared with those who did not.
- Renal function should be monitored, and appropriate adjustment of the immunosuppressive regimen should be considered in patients with elevated or increasing serum creatinine levels.
Sirolimus Tablets
- The safety and efficacy of sirolimus Oral Solution and sirolimus tablets for the prevention of organ rejection following renal transplantation were demonstrated to be clinically equivalent in a randomized, multicenter, controlled trial.
Cyclosporine Withdrawal Study
- The safety and efficacy of sirolimus as a maintenance regimen were assessed following cyclosporine withdrawal at 3 to 4 months after renal transplantation. Study 3 was a randomized, multicenter, controlled trial conducted at 57 centers in Australia, Canada, and Europe. Five hundred twenty-five (525) patients were enrolled. All patients in this study received the tablet formulation.
- This study compared patients who were administered sirolimus, cyclosporine, and corticosteroids continuously with patients who received this same standardized therapy for the first 3 months after transplantation (pre-randomization period) followed by the withdrawal of cyclosporine. During cyclosporine withdrawal, the sirolimus dosages were adjusted to achieve targeted sirolimus whole blood trough concentration ranges (16 to 24 ng/mL until month 12, then 12 to 20 ng/mL thereafter, expressed as chromatographic assay values). At 3 months, 430 patients were equally randomized to either continue sirolimus with cyclosporine therapy or to receive sirolimus as a maintenance regimen following cyclosporine withdrawal.
- Eligibility for randomization included no Banff Grade 3 acute rejection or vascular rejection episode in the 4 weeks before random assignment, serum creatinine ≤ 4.5 mg/dL, and adequate renal function to support cyclosporine withdrawal (in the opinion of the investigator). The primary efficacy endpoint was graft survival at 12 months after transplantation. Secondary efficacy endpoints were the rate of biopsy-confirmed acute rejection, patient survival, incidence of efficacy failure (defined as the first occurrence of either biopsy-proven acute rejection, graft loss, or death), and treatment failure (defined as the first occurrence of either discontinuation, acute rejection, graft loss, or death).
- The following TABLE summarizes the resulting graft and patient survival at 12, 24, and 36 months for this trial. At 12, 24, and 36 months, graft and patient survival were similar for both groups.
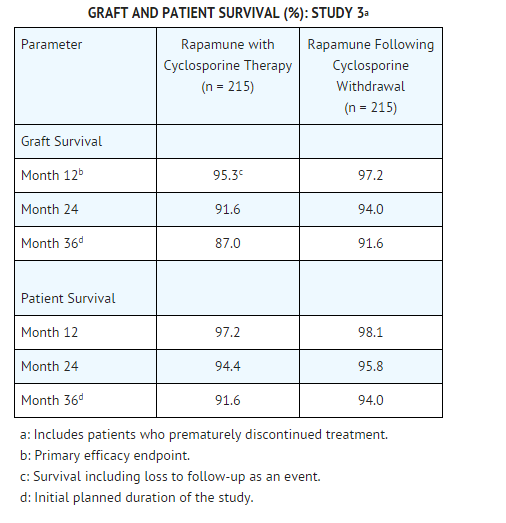
This image is provided by the National Library of Medicine. 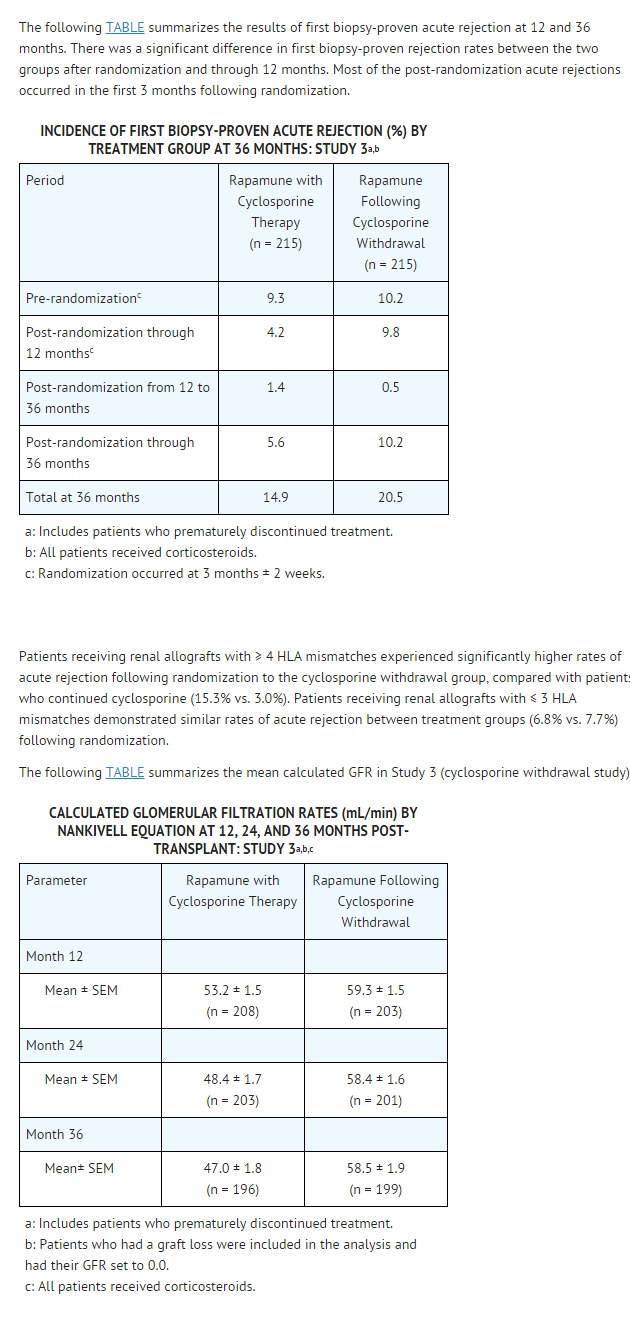
This image is provided by the National Library of Medicine.
- The mean GFR at 12, 24, and 36 months, calculated by the Nankivell equation, was significantly higher for patients receiving sirolimus as a maintenance regimen following cyclosporine withdrawal than for those in the sirolimus with cyclosporine therapy group. Patients who had an acute rejection prior to randomization had a significantly higher GFR following cyclosporine withdrawal compared to those in the sirolimus with cyclosporine group. There was no significant difference in GFR between groups for patients who experienced acute rejection post-randomization.
- Although the initial protocol was designed for 36 months, there was a subsequent amendment to extend this study. The results for the cyclosporine withdrawal group at months 48 and 60 were consistent with the results at month 36. Fifty-two percent (112/215) of the patients in the sirolimus with cyclosporine withdrawal group remained on therapy to month 60 and showed sustained GFR.
High-Immunologic Risk Patients
- Sirolimus was studied in a one-year, clinical trial in high risk patients (Study 4) who were defined as Black transplant recipients and/or repeat renal transplant recipients who lost a previous allograft for immunologic reasons and/or patients with high panel-reactive antibodies (PRA; peak PRA level > 80%). Patients received concentration-controlled sirolimus and cyclosporine (MODIFIED), and corticosteroids per local practice. The sirolimus dose was adjusted to achieve target whole blood trough sirolimus concentrationsof 10-15 ng/mL (chromatographic method) throughout the 12-month study period. The cyclosporine dose was adjusted to achieve target whole blood trough concentrations of 200-300 ng/mL through week 2, 150-200 ng/mL from week 2 to week 26, and 100-150 ng/mL from week 26 to week 52 for the observed trough concentrations ranges. Antibody induction was allowed per protocol as prospectively defined at each transplant center, and was used in 88.4% of patients. The study was conducted at 35 centers in the United States.
- A total of 224 patients received a transplant and at least one dose of sirolimus and cyclosporine and was comprised of 77.2% Black patients, 24.1% repeat renal transplant recipients, and 13.5% patients with high PRA. Efficacy was assessed with the following endpoints, measured at 12 months: efficacy failure (defined as the first occurrence of biopsy-confirmed acute rejection, graft loss, or death), first occurrence of graft loss or death, and renal function as measured by the calculated GFR using the Nankivell formula. The TABLE below summarizes the result of these endpoints.
- Patient survival at 12 months was 94.6%. The incidence of biopsy-confirmed acute rejection was 17.4% and the majority of the episodes of acute rejection were mild in severity.
Conversion from Calcineurin Inhibitors to Sirolimus in Maintenance Renal Transplant Patients
- Conversion from calcineurin inhibitors (CNI) to sirolimus was assessed in maintenance renal transplant patients 6 months to 10 years post‑transplant (Study 5). This study was a randomized, multicenter, controlled trial conducted at 111 centers globally, including US and Europe, and was intended to show that renal function was improved by conversion from CNI to sirolimus. Eight hundred thirty (830) patients were enrolled and stratified by baseline calculated glomerular filtration rate (GFR, 20-40 mL/min vs. greater than 40 mL/min). In this trial there was no benefit associated with conversion with regard to improvement in renal function and a greater incidence of proteinuria in the sirolimus conversion arm. In addition, enrollment of patients with baseline calculated GFR less than 40 mL/min was discontinued due to a higher rate of serious adverse events, including pneumonia, acute rejection, graft loss and death.
- This study compared renal transplant patients (6-120 months after transplantation) who were converted from calcineurin inhibitors to sirolimus, with patients who continued to receive calcineurin inhibitors. Concomitant immunosuppressive medications included mycophenolate mofetil (MMF), azathioprine (AZA), and corticosteroids. sirolimus was initiated with a single loading dose of 12-20 mg, after which dosing was adjusted to achieve a target sirolimus whole blood trough concentration of 8-20 ng/mL (chromatographic method). The efficacy endpoint was calculated GFR at 12 months post-randomization. Additional endpoints included biopsy-confirmed acute rejection, graft loss, and death. Findings in the patient stratum with baseline calculated GFR greater than 40 mL/min (sirolimus conversion, n = 497; CNI continuation, n = 246) are summarized BELOW: There was no clinically or statistically significant improvement in Nankivell GFR compared to baseline.
- The rates of acute rejection, graft loss, and death were similar at 1 and 2 years. Treatment-emergent adverse events occurred more frequently during the first 6 months after sirolimus conversion. The rates of pneumonia were significantly higher for the sirolimus conversion group.
- While the mean and median values for urinary protein to creatinine ratio were similar between treatment groups at baseline, significantly higher mean and median levels of urinary protein excretion were seen in the sirolimus conversion arm at 1 year and at 2 years, as shown in the TABLE below.
- In addition, when compared to patients who continued to receive calcineurin inhibitors, a higher percentage of patients had urinary protein to creatinine ratios > 1 at 1 and 2 years after sirolimus conversion. This difference was seen in both patients who had a urinary protein to creatinine ratio ≤ 1 and those who had a protein to creatinine ratio > 1 at baseline. More patients in the sirolimus conversion group developed nephrotic range proteinuria, as defined by a urinary protein to creatinine ratio > 3.5 (46/482 [9.5%] vs. 9/239 [3.8%]), even when the patients with baseline nephrotic range proteinuria were excluded. The rate of nephrotic range proteinuria was significantly higher in the sirolimus conversion group compared to the calcineurin inhibitor continuation group with baseline urinary protein to creatinine ratio > 1 (13/29 vs. 1/14), excluding patients with baseline nephrotic range proteinuria.
- The above information should be taken into account when considering conversion from calcineurin inhibitors to sirolimus in stable renal transplant patients due to the lack of evidence showing that renal function improves following conversion, and the finding of a greater increment in urinary protein excretion, and an increased incidence of treatment-emergent nephrotic range proteinuria following conversion to sirolimus.
- This was particularly true among patients with existing abnormal urinary protein excretion prior to conversion.
Pediatrics
- Sirolimus was evaluated in a 36-month, open-label, randomized, controlled clinical trial at 14 North American centers in pediatric (aged 3 to < 18 years) renal transplant patients considered to be at high-immunologic risk for developing chronic allograft nephropathy, defined as a history of one or more acute allograft rejection episodes and/or the presence of chronic allograft nephropathy on a renal biopsy. Seventy-eight (78) subjects were randomized in a 2:1 ratio to sirolimus (sirolimus target concentrations of 5 to 15 ng/mL, by chromatographic assay, n = 53) in combination with a calcineurin inhibitor and corticosteroids or to continue calcineurin-inhibitor-based immunosuppressive therapy (n = 25). The primary endpoint of the study was efficacy failure as defined by the first occurrence of biopsy-confirmed acute rejection, graft loss, or death, and the trial was designed to show superiority of sirolimus added to a calcineurin-inhibitor-based immunosuppressive regimen compared to a calcineurin-inhibitor-based regimen.
- The cumulative incidence of efficacy failure up to 36 months was 45.3% in the sirolimus group compared to 44.0% in the control group, and did not demonstrate superiority. There was one death in each group. The use of sirolimus in combination with calcineurin inhibitors and corticosteroids was associated with an increased risk of deterioration of renal function, serum lipid abnormalities (including, but not limited to, increased serum triglycerides and cholesterol), and urinary tract infections . This study does not support the addition of sirolimus to calcineurin-inhibitor-based immunosuppressive therapy in this subpopulation of pediatric renal transplant patients.
How Supplied
- Since sirolimus is not absorbed through the skin, there are no special precautions. However, if direct contact with the skin or mucous membranes occurs, wash thoroughly with soap and water; rinse eyes with plain water.
Sirolimus Oral Solution
- Each sirolimus Oral Solution carton, NDC 0008-1030-06, contains one 2 oz (60 mL fill) amber glass bottle of sirolimus (concentration of 1 mg/mL), one oral syringe adapter for fitting into the neck of the bottle, sufficient disposable amber oral syringes and caps for daily dosing, and a carrying case.
- Sirolimus Oral Solution bottles should be stored protected from light and refrigerated at 2°C to 8°C (36°F to 46°F). Once the bottle is opened, the contents should be used within one month. If necessary, the patient may store the bottles at room temperatures up to 25°C (77°F) for a short period of time (e.g., not more than 15 days for the bottles).
- An amber syringe and cap are provided for dosing, and the product may be kept in the syringe for a maximum of 24 hours at room temperatures up to 25°C (77°F) or refrigerated at 2°C to 8°C (36°F to 46°F). The syringe should be discarded after one use. After dilution, the preparation should be used immediately.
- Sirolimus Oral Solution provided in bottles may develop a slight haze when refrigerated. If such a haze occurs, allow the product to stand at room temperature and shake gently until the haze disappears. The presence of this haze does not affect the quality of the product.
Sirolimus Tablets
- Sirolimus Tablets are available as follows:
- NDC 0008-1041-05, 1 mg, white, triangular-shaped tablets marked"Rapamune 1 mg” on one side; bottle containing 100 tablets.
- NDC 0008-1041-10, 1 mg, white, triangular-shaped tablets marked“RAPAMUNE 1 mg” on one side; in Redipak® cartons of 100 tablets (10 blister cards of 10 tablets each).
- NDC 0008-1042-05, 2 mg, yellow-to-beige triangular-shaped tablets marked “ Rapamune 2 mg” on one side; bottle containing 100 tablets.
Storage
- Rapamune Tablets should be stored at 20° to 25°C [USP Controlled Room Temperature] (68° to 77°F). Use cartons to protect blister cards and strips from light. Dispense in a tight, light-resistant container as defined in the USP.
Images
Drug Images
{{#ask: Page Name::Sirolimus |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sirolimus |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Dosage
- Patients should be given complete dosage instructions.
Skin Cancer Events
- Patients should be told that exposure to sunlight and ultraviolet (UV) light should be limited by wearing protective clothing and using a sunscreen with a high protection factor because of the increased risk for skin cancer.
Pregnancy Risks
- Women of childbearing potential should be informed of the potential risks during pregnancy and told that they should use effective contraception prior to initiation of sirolimus therapy, during sirolimus therapy, and for 12 weeks after sirolimus therapy has been stopped.
Precautions with Alcohol
- Alcohol-Sirolimus interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Rapamune ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "RAPAMUNE- sirolimus tablet, sugar coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Sirolimus |Label Name=Sirolimus 15.jpg
}}
{{#subobject:
|Label Page=Sirolimus |Label Name=Sirolimus 16.png
}}