Sinecatechins: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Sinecatechins": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (5 intermediate revisions by one other user not shown) | |||
| Line 3: | Line 3: | ||
|genericName=Sinecatechins | |genericName=Sinecatechins | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=Keratolytic | |drugClass=[[Keratolytic]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=external genital and perianal warts | |indication=[[Genital warts|external genital]] and [[Wart|perianal warts]] | ||
|adverseReactions=local skin and application site reactions including erythema, pruritus, burning, pain/discomfort, erosion/ulceration, edema, induration, and rash | |adverseReactions=local skin and [[application site reactions]] including [[erythema]], [[pruritus]], [[burning]], [[pain]]/[[discomfort]], [[erosion]]/[[ulceration]], [[edema]], [[induration]], and [[Rash|vesicular rash]] | ||
| Line 20: | Line 20: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=====Indications==== | |fdaLIADAdult=====Indications==== | ||
* | * Sinecatechins is indicated for the topical treatment of [[Genital warts|external genital]] and [[Wart|perianal warts]] ([[Condylomata acuminata]]) in immunocompetent patients 18 years and older. | ||
Limitations of Use | =====Limitations of Use===== | ||
The safety and effectiveness of | * The safety and effectiveness of Sinecatechins have not been established for treatment beyond 16-weeks or for multiple treatment courses. | ||
The safety and effectiveness of | * The safety and effectiveness of Sinecatechins in immunosuppressed patients have not been established. | ||
====Dosage==== | ====Dosage==== | ||
General Dosing Information | =====General Dosing Information===== | ||
* Sinecatechins is to be applied three times per day to all [[Genital warts|external genital]] and [[Wart|perianal warts]]. | |||
Apply about an 0.5 cm strand of the | * Apply about an 0.5 cm strand of the Sinecatechins to each wart using the finger(s), dabbing it on to ensure complete coverage and leaving a thin layer of the ointment on the [[warts]]. Patients should wash their hands before and after application of Sinecatechins. | ||
It is not necessary to wash off the ointment from the treated area prior to the next application. | * It is not necessary to wash off the [[ointment]] from the treated area prior to the next application. | ||
* Sinecatechins is not for ophthalmic, oral, intravaginal, or intra-anal use. | |||
=====Treatment Period===== | |||
Treatment with | * Treatment with Sinecatechins should be continued until complete clearance of all [[warts]], however no longer than 16 weeks. | ||
Local skin reactions (e.g. erythema) at the treatment site are frequent. Nevertheless, treatment should be continued when the severity of the local skin reaction is acceptable. | * Local skin reactions (e.g. [[erythema]]) at the treatment site are frequent. Nevertheless, treatment should be continued when the severity of the local skin reaction is acceptable. | ||
Ointment, 15% w/w. Each gram of | ====DOSAGE FORMS AND STRENGTHS==== | ||
* Ointment, 15% w/w. Each gram of Sinecatechins Ointment, 15% contains 150 mg of sinecatechins in a brown ointment base. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 66: | Line 67: | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings=* Sinecatechins has not been evaluated for the treatment of urethral, intra-vaginal, cervical, rectal, or intra-anal [[Human papillomavirus|human papilloma viral]] disease and should not be used for the treatment of these conditions. | ||
Use of | * Use of Sinecatechins on open wounds should be avoided. | ||
Patients should be advised to avoid exposure of the genital and perianal area to sun/UV-light as | * Patients should be advised to avoid exposure of the genital and perianal area to sun/UV-light as Sinecatechins has not been tested under these circumstances. | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
In Phase 3 clinical trials, a total of 397 subjects received | * In Phase 3 clinical trials, a total of 397 subjects received Sinecatechins three times per day topical application for the treatment of [[Genital warts|external genital]] and [[perianal warts]] for up to 16 weeks. | ||
Serious local adverse events of pain and inflammation were reported in two subjects (0.5%), both women. | * Serious local adverse events of pain and inflammation were reported in two subjects (0.5%), both women. | ||
In clinical trials, the incidence of patients with local adverse events leading to discontinuation or dose interruption (reduction) was 5% (19/397). These included the following events: application site reactions (local pain, erythema, vesicles, skin erosion/ulceration), phimosis, inguinal lymphadenitis, urethral meatal stenosis, dysuria, genital herpes simplex, vulvitis, hypersensitivity, pruritus, pyodermitis, skin ulcer, erosions in the urethral meatus, and superinfection of warts and ulcers. | * In clinical trials, the incidence of patients with local adverse events leading to discontinuation or dose interruption (reduction) was 5% (19/397). These included the following events: [[application site reactions]] (local [[pain]], [[erythema]], [[vesicles]], [[skin erosion]]/[[ulceration]]), [[phimosis]], [[inguinal lymphadenitis]], [[urethral meatal stenosis]], [[dysuria]], [[Herpes simplex|genital herpes simplex]], [[vulvitis]], [[hypersensitivity]], [[pruritus]], [[pyodermitis]], [[skin ulcer]], erosions in the [[urethral meatus]], and superinfection of [[warts]] and [[ulcers]]. | ||
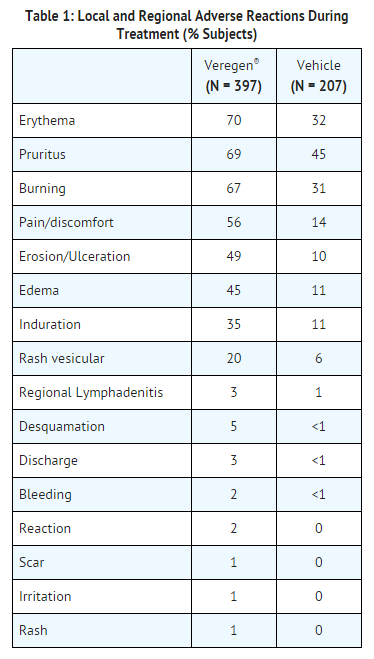
Local and regional reactions (including adenopathy) occurring at >1% in the treated groups are presented in Table 1. | * Local and regional reactions (including [[adenopathy]]) occurring at >1% in the treated groups are presented in Table 1. | ||
: [[File:Sinecatechins Adv Eff.png|none|500px]] | : [[File:Sinecatechins Adv Eff.png|none|500px]] | ||
* A total of 266/397 (67%) of subjects in the | * A total of 266/397 (67%) of subjects in the Sinecatechins group had either a moderate or a severe reaction that was considered probably related to the drug, of which 120 (30%) subjects had a severe reaction. Severe reactions occurred in 37% (71/192) of women and in 24% (49/205) of men. The percentage of subjects with at least one severe, related adverse event was 26% (86/328) for subjects with [[genital warts]] only, 42% (19/45) in subjects with both genital and [[perianal warts]] and 48% (11/23) of subjects with perianal warts only. | ||
Phimosis occurred in 3% of uncircumcised male subjects (5/174) treated with | * [[Phimosis]] occurred in 3% of uncircumcised male subjects (5/174) treated with Sinecatechins and in 1% (1/99) in vehicle. | ||
The maximum mean severity of erythema, erosion, edema, and induration was observed by week 2 of treatment. | * The maximum mean severity of [[erythema]], [[erosion]], [[edema]], and [[induration]] was observed by week 2 of treatment. | ||
Less common local adverse events included urethritis, perianal infection, pigmentation changes, dryness, eczema, hyperesthesia, necrosis, papules, and discoloration. Other less common adverse events included cervical dysplasia, pelvic pain, cutaneous facial rash, and staphylococcemia. | * Less common local adverse events included [[urethritis]], [[perianal infection]], [[pigmentation changes]], [[dryness]], [[eczema]], [[hyperesthesia]], [[necrosis]], [[papules]], and [[discoloration]]. Other less common adverse events included [[cervical dysplasia]], [[pelvic pain]], [[cutaneous facial rash]], and [[staphylococcemia]]. | ||
In a dermal sensitization study of | * In a dermal sensitization study of Sinecatechins in healthy volunteers, [[hypersensitivity]] (type IV) was observed in 5 out of 209 subjects (2.4%) under occlusive conditions. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions=<!--Use in Specific Populations--> | ||
<!--Use in Specific Populations--> | |||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=There are no adequate and well controlled studies in pregnant women. | |useInPregnancyFDA=* There are no adequate and well controlled studies in pregnant women. Sinecatechins should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | ||
The Maximum Recommended Human Dose (MRHD) of | * The Maximum Recommended Human Dose (MRHD) of Sinecatechins was set at three times daily topical administration of 250 mg, 750 mg total, containing 112.5 mg sinecatechins for the animal multiple of human exposure calculations presented in this labelling. Dose multiples were calculated based on the human equivalent dose (HED). | ||
Embryo-fetal development studies were conducted in rats and rabbits using intravaginal and systemic routes of administration, respectively. Oral administration of sinecatechins during the period of organogenesis (gestational Days 6 to 15 in rats or 6 to 18 in rabbits) did not cause treatment related effects on embryo-fetal development or teratogenicity at doses of up to 1,000 mg/kg/day (86-fold MRHD in rats; 173-fold MRHD in rabbits). | * Embryo-fetal development studies were conducted in rats and rabbits using [[intravaginal]] and systemic routes of administration, respectively. Oral administration of sinecatechins during the period of [[organogenesis]] (gestational Days 6 to 15 in rats or 6 to 18 in rabbits) did not cause treatment related effects on embryo-fetal development or teratogenicity at doses of up to 1,000 mg/kg/day (86-fold MRHD in rats; 173-fold MRHD in rabbits). | ||
In the presence of maternal toxicity (characterized by marked local irritation at the administration sites and decreased body weight and food consumption) in pregnant female rabbits, subcutaneous doses of 12 and 36 mg/kg/day of sinecatechins during the period of organogenesis (gestational Days 6 to 19) resulted in corresponding influences on fetal development including reduced fetal body weights and delays in skeletal ossification. No treatment related effects on embryo-fetal development were noted at 4 mg/kg/day (0.7-fold MRHD). There was no evidence of teratogenic effects at any of the doses evaluated in this study. | * In the presence of maternal toxicity (characterized by marked local irritation at the administration sites and decreased body weight and food consumption) in pregnant female rabbits, [[subcutaneous]] doses of 12 and 36 mg/kg/day of sinecatechins during the period of [[organogenesis]] (gestational Days 6 to 19) resulted in corresponding influences on fetal development including reduced fetal body weights and delays in skeletal ossification. No treatment related effects on embryo-fetal development were noted at 4 mg/kg/day (0.7-fold MRHD). There was no evidence of teratogenic effects at any of the doses evaluated in this study. | ||
A combined fertility / embryo-fetal development study using daily vaginal administration of | * A combined fertility / embryo-fetal development study using daily vaginal administration of Sinecatechins to rats from Day 4 before mating and throughout mating until Day 17 of gestation did not show treatment-related effects on embryo-fetal development or [[teratogenicity]] at doses up to 0.15 mL/rat/day (8-fold MRHD). | ||
A pre- and post-natal development study was conducted in rats using vaginal administration of | * A pre- and post-natal development study was conducted in rats using vaginal administration of Sinecatechins at doses of 0.05, 0.10 and 0.15 mL/rat/day from Day 6 of gestation through parturition and lactation. The high and intermediate dose levels of 0.15 (8-fold MRHD) and 0.10 mL/rat/day resulted in an increased mortality of the F0 dams, associated with indications of parturition complications. The high dose level of 0.15 mL/rat/day also resulted in an increased incidence of stillbirths. There were no other treatment-related effects on pre- and post-natal development, growth, reproduction and fertility at any dose tested. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=* It is not known whether topically applied | |useInNursing=* It is not known whether topically applied Sinecatechins is excreted in breast milk. | ||
|useInPed=* Safety and effectiveness in pediatric patients have not been established. | |useInPed=* Safety and effectiveness in pediatric patients have not been established. | ||
|useInGeri=* Seven patients (1.4%), older than 65 years of age were treated with | |useInGeri=* Seven patients (1.4%), older than 65 years of age were treated with Sinecatechins in clinical studies. This, however, is an insufficient number of subjects to determine whether they respond differently from younger subjects. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
| Line 192: | Line 142: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox=<!--Mechanism of Action--> | |drugBox=<!--Mechanism of Action--> | ||
|mechAction=* The mode of action of | |mechAction=* The mode of action of Sinecatechins involved in the clearance of genital and perianal warts is unknown. In vitro, sinecatechins had anti-oxidative activity; the clinical significance of this finding is unknown. | ||
<!--Structure--> | <!--Structure--> | ||
|structure=* | |structure=* Sinecatechins (sinecatechins) Ointment, 15% is a botanical drug product for topical use. The drug substance in Sinecatechins is sinecatechins, which is a partially purified fraction of the water extract of green tea leaves from [[Camellia sinensis]] (L.) O Kuntze, and is a mixture of catechins and other green tea components. [[Catechins]] constitute 85 to 95% (by weight) of the total drug substance which includes more than 55% of [[Epigallocatechin gallate]] (EGCg), other catechin derivatives such as [[Epicatechin]] (EC), [[Epigallocatechin]] (EGC), [[Epicatechin gallate]] (ECg), and some additional minor catechin derivatives i.e. [[Gallocatechin gallate]] (GCg), [[Gallocatechin]] (GC), [[Catechin gallate]] (Cg), and Catechin (C). In addition to the known catechin components, it also contains gallic acid, caffeine, and theobromine which together constitute about 2.5% of the drug substance. The remaining amount of the drug substance contains undefined botanical constituents derived from green tea leaves. | ||
The structural formulae of catechins are shown below. | * The structural formulae of catechins are shown below. | ||
: [[File:Sinecatechins Str.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sinecatechins Str.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Each gram of the ointment contains 150 mg of sinecatechins in a water free ointment base consisting of isopropyl myristate, white petrolatum, cera alba (white wax), propylene glycol palmitostearate, and oleyl alcohol. | * Each gram of the ointment contains 150 mg of sinecatechins in a water free ointment base consisting of [[isopropyl myristate]], white petrolatum, cera alba (white wax), propylene glycol palmitostearate, and oleyl alcohol. | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=* The pharmacodynamics of | |PD=* The pharmacodynamics of Sinecatechins is unknown. | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=* Systemic exposure to EGCg, EGC, ECg, and EC were evaluated following either topical application of | |PK=* Systemic exposure to EGCg, EGC, ECg, and EC were evaluated following either topical application of Sinecatechins to subjects with [[Genital warts|external genital]] and [[Warts|perianal warts]] (250 mg applied 3 times a day for 7 days) or following oral ingestion of green tea beverage (500 mL ingested 3 times a day for 7 days). Following topical application of Sinecatechins, plasma concentration of all 4 catechins were below the limit of quantification (<5 ng/mL) on Day 1. After application of Sinecatechins for 7 days, plasma EGC, ECg, and EC concentrations were below the limit of quantification while plasma concentration of EGCg were measurable in 2 out of 20 subjects. The mean maximal plasma concentration (Cmax) of EGCg was 10.1 ng/mL and the mean area under the concentration versus time curve (AUC) of EGCg was 52.2 ng*h/mL in these 2 subjects. Oral ingestion of green tea beverage resulted in measurable concentration of EGCg in all subjects on both Day 1 and Day 7, with mean (SD) Cmax of 23.0 (12.0) ng/mL and AUC of 104.6 (39.0) ng*h/mL on Day 7. | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=Carcinogenesis, Mutagenesis, Impairment of Fertility | |nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | ||
In an oral (gavage) carcinogenicity study, sinecatechins was administered daily for 26 weeks to p53 transgenic mice at doses up to 500 mg/kg/day (22-fold MRHD | * In an oral (gavage) carcinogenicity study, sinecatechins was administered daily for 26 weeks to p53 transgenic mice at doses up to 500 mg/kg/day (22-fold MRHD). Treatment with sinecatechins was not associated with an increased incidence of either neoplastic or non-neoplastic lesions in the organs and tissues examined. Sinecatechins has not been evaluated in a dermal carcinogenicity study. | ||
Sinecatechins was negative in the Ames test, in vivo rat micronucleus assay, UDS test, and transgenic mouse mutation assay, but positive in the mouse lymphoma mutation assay. | * Sinecatechins was negative in the [[Ames test]], in vivo rat micronucleus assay, [[UDS test]], and transgenic mouse mutation assay, but positive in the mouse lymphoma mutation assay. | ||
Daily vaginal administration of | * Daily vaginal administration of Sinecatechins to rats from Day 4 before mating and throughout mating until Day 17 of gestation did not cause adverse effects on mating performance and fertility at doses up to 0.15 mL/rat/day. This dose corresponds to approximately 150 mg/rat/day (8-fold MRHD). | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=Two randomized, double-blind, vehicle-controlled trials were performed to investigate the safety and efficacy of | |clinicalStudies=* Two randomized, double-blind, vehicle-controlled trials were performed to investigate the safety and efficacy of Sinecatechins in the treatment of immunocompetent subjects 18 years of age and older with [[Genital warts|external genital]] and [[Warts|perianal warts]]. The subjects applied the ointment 3 times daily for up to 16 weeks or until complete clearance of all warts (baseline and new warts occurring during treatment). | ||
Over both trials the median baseline wart area was 51 mm2 (range 12 to 585 mm2), and the median baseline number of warts was 6 (range 2 to 30). | * Over both trials the median baseline [[wart]] area was 51 mm2 (range 12 to 585 mm2), and the median baseline number of warts was 6 (range 2 to 30). | ||
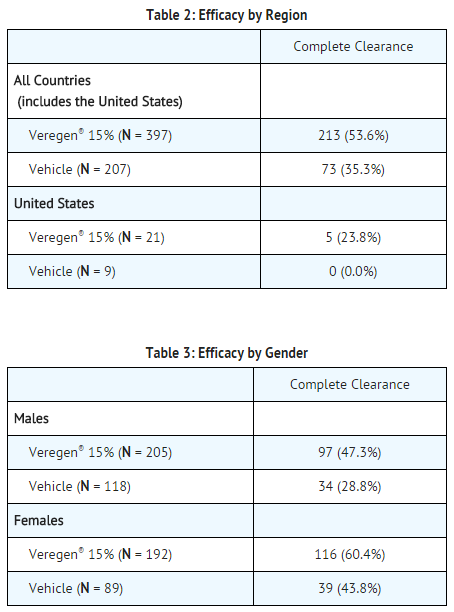
The primary efficacy outcome measure was the response rate defined as the proportion of subjects with complete clinical (visual) clearance of all external genital and perianal warts (baseline and new) by week 16, presented in Tables 2 and 3 for all randomized subjects dispensed medication. | * The primary efficacy outcome measure was the response rate defined as the proportion of subjects with complete clinical (visual) clearance of all [[Genital warts|external genital]] and [[Warts|perianal warts]] (baseline and new) by week 16, presented in Tables 2 and 3 for all randomized subjects dispensed medication. | ||
: [[File:Sinecatechins CS.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:Sinecatechins CS.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 228: | Line 178: | ||
* Median time to complete wart clearance was 16 weeks and 10 weeks, respectively, in the two phase 3 clinical trials. | * Median time to complete wart clearance was 16 weeks and 10 weeks, respectively, in the two phase 3 clinical trials. | ||
The rate of recurrence of external genital and perianal warts 12 weeks after completion of treatment in subjects with complete clearance is 6.8% (14/206) for those treated with | * The rate of recurrence of external genital and perianal warts 12 weeks after completion of treatment in subjects with complete clearance is 6.8% (14/206) for those treated with Sinecatechins and 5.8% (4/69) for those treated with vehicle. | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=* Sinecatechins is a brown ointment and is supplied in an aluminum tube containing 15 grams (NDC # 10337-450-15) of ointment per tube or 30 grams (NDC # 10337-450-03) of ointment per tube. | ||
|storage=* Prior to dispensing to the patient, store refrigerated 2°C to 8°C (36°F to 46°F). After dispensing, store refrigerated or up to 25°C (77°F). Do not freeze. | |storage=* Prior to dispensing to the patient, store refrigerated 2°C to 8°C (36°F to 46°F). After dispensing, store refrigerated or up to 25°C (77°F). Do not freeze. | ||
Keep out of reach of children. | * Keep out of reach of children. | ||
|packLabel=====PRINCIPAL DISPLAY PANEL==== | |||
: [[File:Sinecatechins PDP 1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Sinecatechins PDP 2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Sinecatechins PDP 3.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:Sinecatechins PDP 4.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Ingredients and Appearance==== | |||
: [[File: | : [[File:Sinecatechins Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=Patients using | |fdaPatientInfo=* Patients using Sinecatechins should receive the following information and instructions: | ||
This medication is only to be used as directed by a physician. It is for external use only. Eye contact should be avoided as well as application into the vagina or anus. | :* This medication is only to be used as directed by a physician. It is for external use only. Eye contact should be avoided as well as application into the vagina or anus. | ||
It is not necessary to wash off | :* It is not necessary to wash off Sinecatechins prior to the next application. When the treatment area is washed or a bath is taken, the ointment should be applied afterwards. | ||
It is common for patients to experience local skin reactions such as erythema, erosion, edema, itching, and burning at the site of application. Severe skin reactions can occur and should be promptly reported to the healthcare provider. Should severe local skin reaction occur, the ointment should be removed by washing the treatment area with mild soap and water, and further doses withheld. | :* It is common for patients to experience local skin reactions such as erythema, erosion, edema, itching, and burning at the site of application. Severe skin reactions can occur and should be promptly reported to the healthcare provider. Should severe local skin reaction occur, the ointment should be removed by washing the treatment area with mild soap and water, and further doses withheld. | ||
Sexual (genital, anal or oral) contact should be avoided while the ointment is on the skin, or the ointment should be washed off prior to these activities. | :* Sexual (genital, anal or oral) contact should be avoided while the ointment is on the skin, or the ointment should be washed off prior to these activities. Sinecatechins may weaken condoms and vaginal diaphragms. Therefore, the use in combination with Sinecatechins is not recommended. | ||
Female patients using tampons should insert the tampon before applying the ointment. If the tampon is changed while the ointment is on the skin, accidental application of the ointment into the vagina must be avoided. | :* Female patients using tampons should insert the tampon before applying the ointment. If the tampon is changed while the ointment is on the skin, accidental application of the ointment into the vagina must be avoided. | ||
:* Sinecatechins may stain clothing and bedding. | |||
:* Sinecatechins is not a cure and new warts might develop during or after a course of therapy. If new warts develop during the 16-week treatment period, these should also be treated with Veregen®. | |||
The effect of | :* The effect of Sinecatechins on the transmission of genital/perianal warts is unknown. | ||
Patients should be advised to avoid exposure of the genital and perianal area to sun/UV light as | :* Patients should be advised to avoid exposure of the genital and perianal area to sun/UV light as Sinecatechins has not been tested under these circumstances. | ||
The treatment area should not be bandaged or otherwise covered or wrapped as to be occlusive. | :* The treatment area should not be bandaged or otherwise covered or wrapped as to be occlusive. | ||
Uncircumcised males treating warts under the foreskin should retract the foreskin and clean the area daily. | :* Uncircumcised males treating warts under the foreskin should retract the foreskin and clean the area daily. | ||
====PATIENT PACKAGE INSERT==== | ====PATIENT PACKAGE INSERT==== | ||
| Line 268: | Line 224: | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 17:09, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
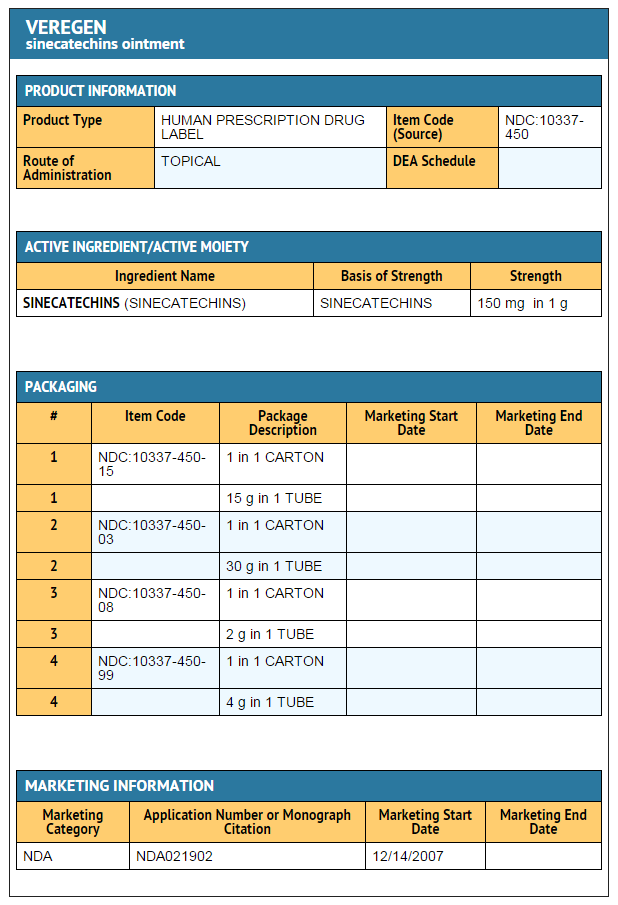
Overview
Sinecatechins is a Keratolytic that is FDA approved for the treatment of external genital and perianal warts. Common adverse reactions include local skin and application site reactions including erythema, pruritus, burning, pain/discomfort, erosion/ulceration, edema, induration, and vesicular rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Sinecatechins is indicated for the topical treatment of external genital and perianal warts (Condylomata acuminata) in immunocompetent patients 18 years and older.
Limitations of Use
- The safety and effectiveness of Sinecatechins have not been established for treatment beyond 16-weeks or for multiple treatment courses.
- The safety and effectiveness of Sinecatechins in immunosuppressed patients have not been established.
Dosage
General Dosing Information
- Sinecatechins is to be applied three times per day to all external genital and perianal warts.
- Apply about an 0.5 cm strand of the Sinecatechins to each wart using the finger(s), dabbing it on to ensure complete coverage and leaving a thin layer of the ointment on the warts. Patients should wash their hands before and after application of Sinecatechins.
- It is not necessary to wash off the ointment from the treated area prior to the next application.
- Sinecatechins is not for ophthalmic, oral, intravaginal, or intra-anal use.
Treatment Period
- Treatment with Sinecatechins should be continued until complete clearance of all warts, however no longer than 16 weeks.
- Local skin reactions (e.g. erythema) at the treatment site are frequent. Nevertheless, treatment should be continued when the severity of the local skin reaction is acceptable.
DOSAGE FORMS AND STRENGTHS
- Ointment, 15% w/w. Each gram of Sinecatechins Ointment, 15% contains 150 mg of sinecatechins in a brown ointment base.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sinecatechins in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sinecatechins in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Sinecatechins in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sinecatechins in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sinecatechins in pediatric patients.
Contraindications
- None
Warnings
- Sinecatechins has not been evaluated for the treatment of urethral, intra-vaginal, cervical, rectal, or intra-anal human papilloma viral disease and should not be used for the treatment of these conditions.
- Use of Sinecatechins on open wounds should be avoided.
- Patients should be advised to avoid exposure of the genital and perianal area to sun/UV-light as Sinecatechins has not been tested under these circumstances.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In Phase 3 clinical trials, a total of 397 subjects received Sinecatechins three times per day topical application for the treatment of external genital and perianal warts for up to 16 weeks.
- Serious local adverse events of pain and inflammation were reported in two subjects (0.5%), both women.
- In clinical trials, the incidence of patients with local adverse events leading to discontinuation or dose interruption (reduction) was 5% (19/397). These included the following events: application site reactions (local pain, erythema, vesicles, skin erosion/ulceration), phimosis, inguinal lymphadenitis, urethral meatal stenosis, dysuria, genital herpes simplex, vulvitis, hypersensitivity, pruritus, pyodermitis, skin ulcer, erosions in the urethral meatus, and superinfection of warts and ulcers.
- Local and regional reactions (including adenopathy) occurring at >1% in the treated groups are presented in Table 1.
- A total of 266/397 (67%) of subjects in the Sinecatechins group had either a moderate or a severe reaction that was considered probably related to the drug, of which 120 (30%) subjects had a severe reaction. Severe reactions occurred in 37% (71/192) of women and in 24% (49/205) of men. The percentage of subjects with at least one severe, related adverse event was 26% (86/328) for subjects with genital warts only, 42% (19/45) in subjects with both genital and perianal warts and 48% (11/23) of subjects with perianal warts only.
- Phimosis occurred in 3% of uncircumcised male subjects (5/174) treated with Sinecatechins and in 1% (1/99) in vehicle.
- The maximum mean severity of erythema, erosion, edema, and induration was observed by week 2 of treatment.
- Less common local adverse events included urethritis, perianal infection, pigmentation changes, dryness, eczema, hyperesthesia, necrosis, papules, and discoloration. Other less common adverse events included cervical dysplasia, pelvic pain, cutaneous facial rash, and staphylococcemia.
- In a dermal sensitization study of Sinecatechins in healthy volunteers, hypersensitivity (type IV) was observed in 5 out of 209 subjects (2.4%) under occlusive conditions.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Sinecatechins in the drug label.
Drug Interactions
There is limited information regarding Sinecatechins Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- There are no adequate and well controlled studies in pregnant women. Sinecatechins should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- The Maximum Recommended Human Dose (MRHD) of Sinecatechins was set at three times daily topical administration of 250 mg, 750 mg total, containing 112.5 mg sinecatechins for the animal multiple of human exposure calculations presented in this labelling. Dose multiples were calculated based on the human equivalent dose (HED).
- Embryo-fetal development studies were conducted in rats and rabbits using intravaginal and systemic routes of administration, respectively. Oral administration of sinecatechins during the period of organogenesis (gestational Days 6 to 15 in rats or 6 to 18 in rabbits) did not cause treatment related effects on embryo-fetal development or teratogenicity at doses of up to 1,000 mg/kg/day (86-fold MRHD in rats; 173-fold MRHD in rabbits).
- In the presence of maternal toxicity (characterized by marked local irritation at the administration sites and decreased body weight and food consumption) in pregnant female rabbits, subcutaneous doses of 12 and 36 mg/kg/day of sinecatechins during the period of organogenesis (gestational Days 6 to 19) resulted in corresponding influences on fetal development including reduced fetal body weights and delays in skeletal ossification. No treatment related effects on embryo-fetal development were noted at 4 mg/kg/day (0.7-fold MRHD). There was no evidence of teratogenic effects at any of the doses evaluated in this study.
- A combined fertility / embryo-fetal development study using daily vaginal administration of Sinecatechins to rats from Day 4 before mating and throughout mating until Day 17 of gestation did not show treatment-related effects on embryo-fetal development or teratogenicity at doses up to 0.15 mL/rat/day (8-fold MRHD).
- A pre- and post-natal development study was conducted in rats using vaginal administration of Sinecatechins at doses of 0.05, 0.10 and 0.15 mL/rat/day from Day 6 of gestation through parturition and lactation. The high and intermediate dose levels of 0.15 (8-fold MRHD) and 0.10 mL/rat/day resulted in an increased mortality of the F0 dams, associated with indications of parturition complications. The high dose level of 0.15 mL/rat/day also resulted in an increased incidence of stillbirths. There were no other treatment-related effects on pre- and post-natal development, growth, reproduction and fertility at any dose tested.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sinecatechins in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sinecatechins during labor and delivery.
Nursing Mothers
- It is not known whether topically applied Sinecatechins is excreted in breast milk.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Seven patients (1.4%), older than 65 years of age were treated with Sinecatechins in clinical studies. This, however, is an insufficient number of subjects to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Sinecatechins with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sinecatechins with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sinecatechins in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sinecatechins in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sinecatechins in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sinecatechins in patients who are immunocompromised.
Administration and Monitoring
Administration
- topical ointment
Monitoring
There is limited information regarding Monitoring of Sinecatechins in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Sinecatechins in the drug label.
Overdosage
There is limited information regarding Overdose of Sinecatechins in the drug label.
Pharmacology
There is limited information regarding Sinecatechins Pharmacology in the drug label.
Mechanism of Action
- The mode of action of Sinecatechins involved in the clearance of genital and perianal warts is unknown. In vitro, sinecatechins had anti-oxidative activity; the clinical significance of this finding is unknown.
Structure
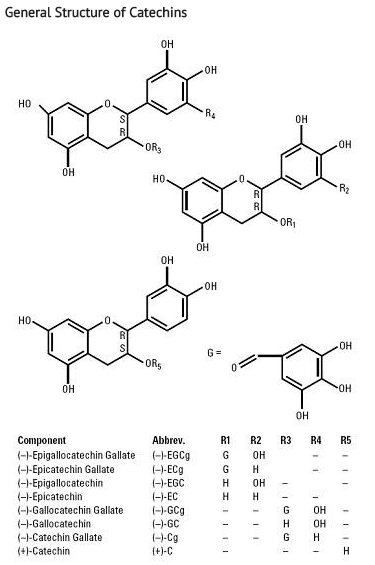
- Sinecatechins (sinecatechins) Ointment, 15% is a botanical drug product for topical use. The drug substance in Sinecatechins is sinecatechins, which is a partially purified fraction of the water extract of green tea leaves from Camellia sinensis (L.) O Kuntze, and is a mixture of catechins and other green tea components. Catechins constitute 85 to 95% (by weight) of the total drug substance which includes more than 55% of Epigallocatechin gallate (EGCg), other catechin derivatives such as Epicatechin (EC), Epigallocatechin (EGC), Epicatechin gallate (ECg), and some additional minor catechin derivatives i.e. Gallocatechin gallate (GCg), Gallocatechin (GC), Catechin gallate (Cg), and Catechin (C). In addition to the known catechin components, it also contains gallic acid, caffeine, and theobromine which together constitute about 2.5% of the drug substance. The remaining amount of the drug substance contains undefined botanical constituents derived from green tea leaves.
- The structural formulae of catechins are shown below.
- Each gram of the ointment contains 150 mg of sinecatechins in a water free ointment base consisting of isopropyl myristate, white petrolatum, cera alba (white wax), propylene glycol palmitostearate, and oleyl alcohol.
Pharmacodynamics
- The pharmacodynamics of Sinecatechins is unknown.
Pharmacokinetics
- Systemic exposure to EGCg, EGC, ECg, and EC were evaluated following either topical application of Sinecatechins to subjects with external genital and perianal warts (250 mg applied 3 times a day for 7 days) or following oral ingestion of green tea beverage (500 mL ingested 3 times a day for 7 days). Following topical application of Sinecatechins, plasma concentration of all 4 catechins were below the limit of quantification (<5 ng/mL) on Day 1. After application of Sinecatechins for 7 days, plasma EGC, ECg, and EC concentrations were below the limit of quantification while plasma concentration of EGCg were measurable in 2 out of 20 subjects. The mean maximal plasma concentration (Cmax) of EGCg was 10.1 ng/mL and the mean area under the concentration versus time curve (AUC) of EGCg was 52.2 ng*h/mL in these 2 subjects. Oral ingestion of green tea beverage resulted in measurable concentration of EGCg in all subjects on both Day 1 and Day 7, with mean (SD) Cmax of 23.0 (12.0) ng/mL and AUC of 104.6 (39.0) ng*h/mL on Day 7.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In an oral (gavage) carcinogenicity study, sinecatechins was administered daily for 26 weeks to p53 transgenic mice at doses up to 500 mg/kg/day (22-fold MRHD). Treatment with sinecatechins was not associated with an increased incidence of either neoplastic or non-neoplastic lesions in the organs and tissues examined. Sinecatechins has not been evaluated in a dermal carcinogenicity study.
- Sinecatechins was negative in the Ames test, in vivo rat micronucleus assay, UDS test, and transgenic mouse mutation assay, but positive in the mouse lymphoma mutation assay.
- Daily vaginal administration of Sinecatechins to rats from Day 4 before mating and throughout mating until Day 17 of gestation did not cause adverse effects on mating performance and fertility at doses up to 0.15 mL/rat/day. This dose corresponds to approximately 150 mg/rat/day (8-fold MRHD).
Clinical Studies
- Two randomized, double-blind, vehicle-controlled trials were performed to investigate the safety and efficacy of Sinecatechins in the treatment of immunocompetent subjects 18 years of age and older with external genital and perianal warts. The subjects applied the ointment 3 times daily for up to 16 weeks or until complete clearance of all warts (baseline and new warts occurring during treatment).
- Over both trials the median baseline wart area was 51 mm2 (range 12 to 585 mm2), and the median baseline number of warts was 6 (range 2 to 30).
- The primary efficacy outcome measure was the response rate defined as the proportion of subjects with complete clinical (visual) clearance of all external genital and perianal warts (baseline and new) by week 16, presented in Tables 2 and 3 for all randomized subjects dispensed medication.
- Median time to complete wart clearance was 16 weeks and 10 weeks, respectively, in the two phase 3 clinical trials.
- The rate of recurrence of external genital and perianal warts 12 weeks after completion of treatment in subjects with complete clearance is 6.8% (14/206) for those treated with Sinecatechins and 5.8% (4/69) for those treated with vehicle.
How Supplied
- Sinecatechins is a brown ointment and is supplied in an aluminum tube containing 15 grams (NDC # 10337-450-15) of ointment per tube or 30 grams (NDC # 10337-450-03) of ointment per tube.
Storage
- Prior to dispensing to the patient, store refrigerated 2°C to 8°C (36°F to 46°F). After dispensing, store refrigerated or up to 25°C (77°F). Do not freeze.
- Keep out of reach of children.
Images
Drug Images
{{#ask: Page Name::Sinecatechins |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Ingredients and Appearance
{{#ask: Label Page::Sinecatechins |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
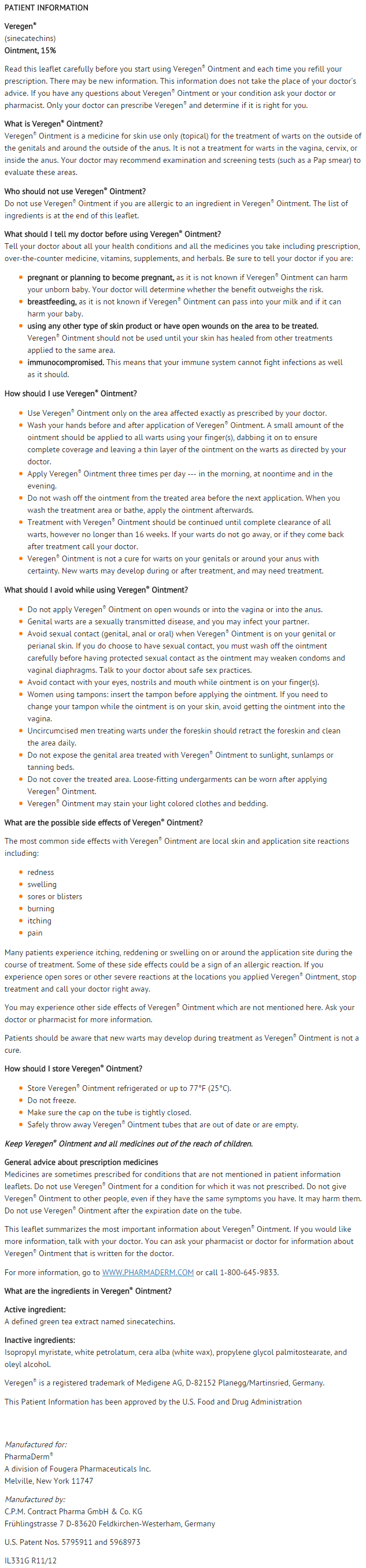
Patient Counseling Information
- Patients using Sinecatechins should receive the following information and instructions:
- This medication is only to be used as directed by a physician. It is for external use only. Eye contact should be avoided as well as application into the vagina or anus.
- It is not necessary to wash off Sinecatechins prior to the next application. When the treatment area is washed or a bath is taken, the ointment should be applied afterwards.
- It is common for patients to experience local skin reactions such as erythema, erosion, edema, itching, and burning at the site of application. Severe skin reactions can occur and should be promptly reported to the healthcare provider. Should severe local skin reaction occur, the ointment should be removed by washing the treatment area with mild soap and water, and further doses withheld.
- Sexual (genital, anal or oral) contact should be avoided while the ointment is on the skin, or the ointment should be washed off prior to these activities. Sinecatechins may weaken condoms and vaginal diaphragms. Therefore, the use in combination with Sinecatechins is not recommended.
- Female patients using tampons should insert the tampon before applying the ointment. If the tampon is changed while the ointment is on the skin, accidental application of the ointment into the vagina must be avoided.
- Sinecatechins may stain clothing and bedding.
- Sinecatechins is not a cure and new warts might develop during or after a course of therapy. If new warts develop during the 16-week treatment period, these should also be treated with Veregen®.
- The effect of Sinecatechins on the transmission of genital/perianal warts is unknown.
- Patients should be advised to avoid exposure of the genital and perianal area to sun/UV light as Sinecatechins has not been tested under these circumstances.
- The treatment area should not be bandaged or otherwise covered or wrapped as to be occlusive.
- Uncircumcised males treating warts under the foreskin should retract the foreskin and clean the area daily.
PATIENT PACKAGE INSERT
Precautions with Alcohol
- Alcohol-Sinecatechins interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Veregen®[1]
Look-Alike Drug Names
There is limited information regarding Sinecatechins Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.