Sarcoidosis differential diagnosis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Sarcoidosis}} | {{Sarcoidosis}} | ||
{{CMG}} {{AE}}Roshan Dinparasti Saleh M.D. | {{CMG}} {{AE}}{{HK}}Roshan Dinparasti Saleh M.D. | ||
==Overview== | ==Overview== | ||
Latest revision as of 21:58, 27 October 2019
|
Sarcoidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sarcoidosis differential diagnosis On the Web |
|
American Roentgen Ray Society Images of Sarcoidosis differential diagnosis |
|
Risk calculators and risk factors for Sarcoidosis differential diagnosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2]Roshan Dinparasti Saleh M.D.
Overview
Sarcoidosis has many differentials, which can be classified depending on the organ involved, pathologic findings and laboratory findings.
Differential Diagnosis
Sarcoidosis has been defined as a multisystem granulomatous disorder of unknown cause[1], but granulomatous inflammation alone is not sufficient for the diagnosis of sarcoidosis because alternative etiologies of granulomatous inflammation need to be excluded.
Causes of granulomatous reaction beside sarcoidosis
- Mycobacterium tuberculosis
- Mycoplasma
- Pneumocystis jiroveci
- Brucellosis
- Cat-scratch fever
- Atypical mycobacteria
- Toxoplasmosis
- Berylliosis
- Hard metal
- Zirconium
- Tattoo
- Hypersensitivity pneumonitis
- Medications (e.g., methotrexate)
- ANCA-associated vasculitis
- Necrotizing sarcoid granuloma
- Lymphoma
- Cancer
- Granulomatous lesions of unknown significance
- Crohn’s disease
- Lymphocytic interstitial pneumonia
- Behçet’s disease
- Rheumatoid nodules[1]
Data supporting the likelihood of sarcoidosis
- Demographics
- Medical history
- Non-smoker
- No symptoms (in patient with bilateral hilar adenopathy on CXR)
- Family history of sarcoidosis
- Symptoms involving more than two organs commonly involved by sarcoidosis
- Laboratory data
- Elevated ACE level (especially if > 2× ULN)
- Elevated calcium level
- Elevated alkaline phosphatase level
- Elevated soluble IL-2 receptor
- Leukopenia
- Radiographic findings
- CXR:
- bilateral hilar adenopathy especially if without symptoms
- Upper lobe disease
- HRCT:
- Disease along bronchovascular bundle
- Subpleural reticulonodular infiltrates
- Mediastinal adenopathy
- Peribronchial thickening
- Traction bronchiectasis of upper lobe
- CXR:
- Skin lesions
- Lupus pernio
- Erythema nodosum
- Maculopapular lesions
- Ocular disease
- Neurological disease
- Renal disease
Data weakening the likelihood of sarcoidosis
- Demographics
- Age< 18 years
- Medical history
- Exposure to tuberculosis
- Exposure to organic bioaerosol
- Exposure to beryllium
- Intravenous drug abuse
- Laboratory data
- Positive anti-neutrophil cytoplasm antibody (ANCA)
- Radiographic findings
- CXR:
- HRCT:
- Subpleural honeycombing
- Ocular disease
- Renal disease
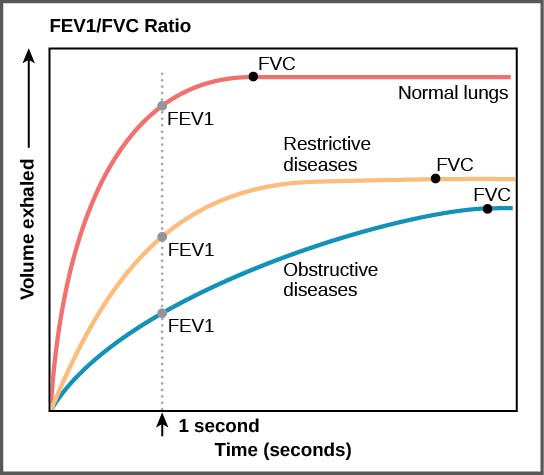
| Spirometry | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Low FEV1/FVC ratio | Normal to high FEV1/FVC ratio | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Obstructive Lung Disease | Restrictive Lung Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bronchodilator therapy | DLCO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increased FEV1 | No change in FEV1 | Normal DLCO | Decreased DLCO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Asthma | COPD | Chest wall disorders | Interstitial Lung Disease | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spirometry Findings in Various Lung Conditions
| Disease | Clinical manifestations | Diagnosis | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | Physical exam | Lab findings | Imaging | Gold standard | |||||||||||||
| Cough | Dyspnea | Hemoptysis | Fever | History/Exposure | Cyanosis | Clubbing | JVD | Peripheral edema | Auscultation | Other prominent findings | CXR | CT | DLCco | ||||
| Hypersensitivity Pneumonitis | + | + | - | + |
|
- | + | - | - |
|
|
|
|
|
↓ |
| |
| Acute Respiratory Distress Syndrome (ARDS) | - | + | - | - | + | - | - | - |
|
|
|
|
|
↓ |
| ||
| Bronchitis | Acute | + | - | +/- | + | - | - | - | - | - |
|
|
|
- |
| ||
| Chronic | + | + | - | - |
|
+ | - | + | + |
|
|
|
|
↓ |
| ||
| Pneumoconiosis[3] | SIlicosis[4][5] | + | + | +/- | - |
|
+ | + | + | - |
|
|
|
|
|
↓ | |
| Asbestosis |
|
|
| ||||||||||||||
| Berylliosis |
|
- |
|
| |||||||||||||
| Byssinosis |
|
|
|
| |||||||||||||
| Sarcoidosis | + | + | + | + |
|
- | - | - | - |
|
|
|
|
|
↓ | ||
| Pleural Effusion | + | + | +/- | +/- | Transudate
Exudate |
+/- | +/- | +/- | +/- |
|
|
|
Supine
Lateral decubitus
|
|
↓ | ||
| Interstitial (Nonidiopathic) Pulmonary Fibrosis | + | ++ | + | - | + | + | + | + |
|
|
|
↓ | Video-assisted thoracoscopic lung biopsy | ||||
| Lymphocytic Interstitial Pneumonia[6] | + | + | + | + | - | + | - | - |
|
|
|
|
N | Open lung biopsy | |||
| Obesity[7][8] | + | + | - | - |
|
- | - | - | + | - |
|
|
N | Clinical | |||
| Pulmonary Eosinophilia[9] | + | + | + | + | Infections | + | - | + | + |
|
|
|
|
↓ | Biopsy of lesion (skin or lung) | ||
| Neuromuscular disease | Scoliosis | - | + | - | - |
|
- | - | - | - |
|
|
|
|
|
N |
|
| Muscular dystrophy | - | + | - | - |
|
- | - | - | - |
|
|
|
N | ||||
| ALS | - | + | - | - |
|
- | - | - | - |
|
|
N/A | Not significant/diagnostic | Not significant/diagnostic | - |
| |
| Myasthenia gravis | - | + | - | + | H/O of difficulty getting up from chair
|
- | - | - | - |
|
|
|
|
|
N | ||
References
- ↑ 1.0 1.1 Statement on sarcoidosis: Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 160:736–755, 1999.
- ↑ 2.0 2.1 Judson MA: The diagnosis of sarcoidosis. Clin Chest Med 29(3):415–427, 2008.
- ↑ Gay SE, Kazerooni EA, Toews GB, Lynch JP, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, Popovich J, Hyzy R, Martinez FJ (1998). "Idiopathic pulmonary fibrosis: predicting response to therapy and survival". Am. J. Respir. Crit. Care Med. 157 (4 Pt 1): 1063–72. doi:10.1164/ajrccm.157.4.9703022. PMID 9563720.
- ↑ du Bois RM (2006). "Evolving concepts in the early and accurate diagnosis of idiopathic pulmonary fibrosis". Clin. Chest Med. 27 (1 Suppl 1): S17–25, v–vi. doi:10.1016/j.ccm.2005.08.001. PMID 16545629.
- ↑ Neghab M, Mohraz MH, Hassanzadeh J (2011). "Symptoms of respiratory disease and lung functional impairment associated with occupational inhalation exposure to carbon black dust". J Occup Health. 53 (6): 432–8. PMID 21996929.
- ↑ Honda O, Johkoh T, Ichikado K, Tomiyama N, Maeda M, Mihara N, Higashi M, Hamada S, Naito H, Yamamoto S, Nakamura H (1999). "Differential diagnosis of lymphocytic interstitial pneumonia and malignant lymphoma on high-resolution CT". AJR Am J Roentgenol. 173 (1): 71–4. doi:10.2214/ajr.173.1.10397102. PMID 10397102.
- ↑ Zammit C, Liddicoat H, Moonsie I, Makker H (2010). "Obesity and respiratory diseases". Int J Gen Med. 3: 335–43. doi:10.2147/IJGM.S11926. PMC 2990395. PMID 21116339.
- ↑ O’Neill, Donal (2015). "Measuring obesity in the absence of a gold standard". Economics & Human Biology. 17: 116–128. doi:10.1016/j.ehb.2015.02.002. ISSN 1570-677X.
- ↑ de Górgolas M, Casado V, Renedo G, Alen JF, Fernández Guerrero ML (2009). "Nodular lung schistosomiais lesions after chemotherapy for dysgerminoma". Am. J. Trop. Med. Hyg. 81 (3): 424–7. PMID 19706907.