Rotavirus
| style="background:#Template:Taxobox colour;"|Rotavirus | ||||||||
|---|---|---|---|---|---|---|---|---|
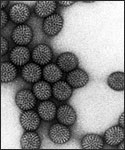 Electron micrograph of Rotaviruses. The bar = 100 nm
| ||||||||
| style="background:#Template:Taxobox colour;" | Virus classification | ||||||||
|
Template:DiseaseDisorder infobox
|
Rotavirus infection Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rotavirus On the Web |
|
American Roentgen Ray Society Images of Rotavirus |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Rotaviruses are a genus of viruses belonging to the Reoviridae family. Seven major groups have been identified, three of which (groups A, B, and C) infect humans, with group A being the most common and widespread one. They cause vomiting and diarrhea and are the most common cause of severe diarrhea in children, killing about 600,000 children every year in developing countries (as of 2005). New vaccines have been shown to be safe and effective in 2006 [2].
Microbiology
Structure
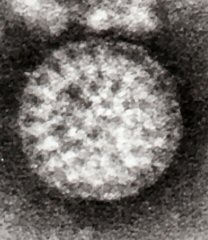
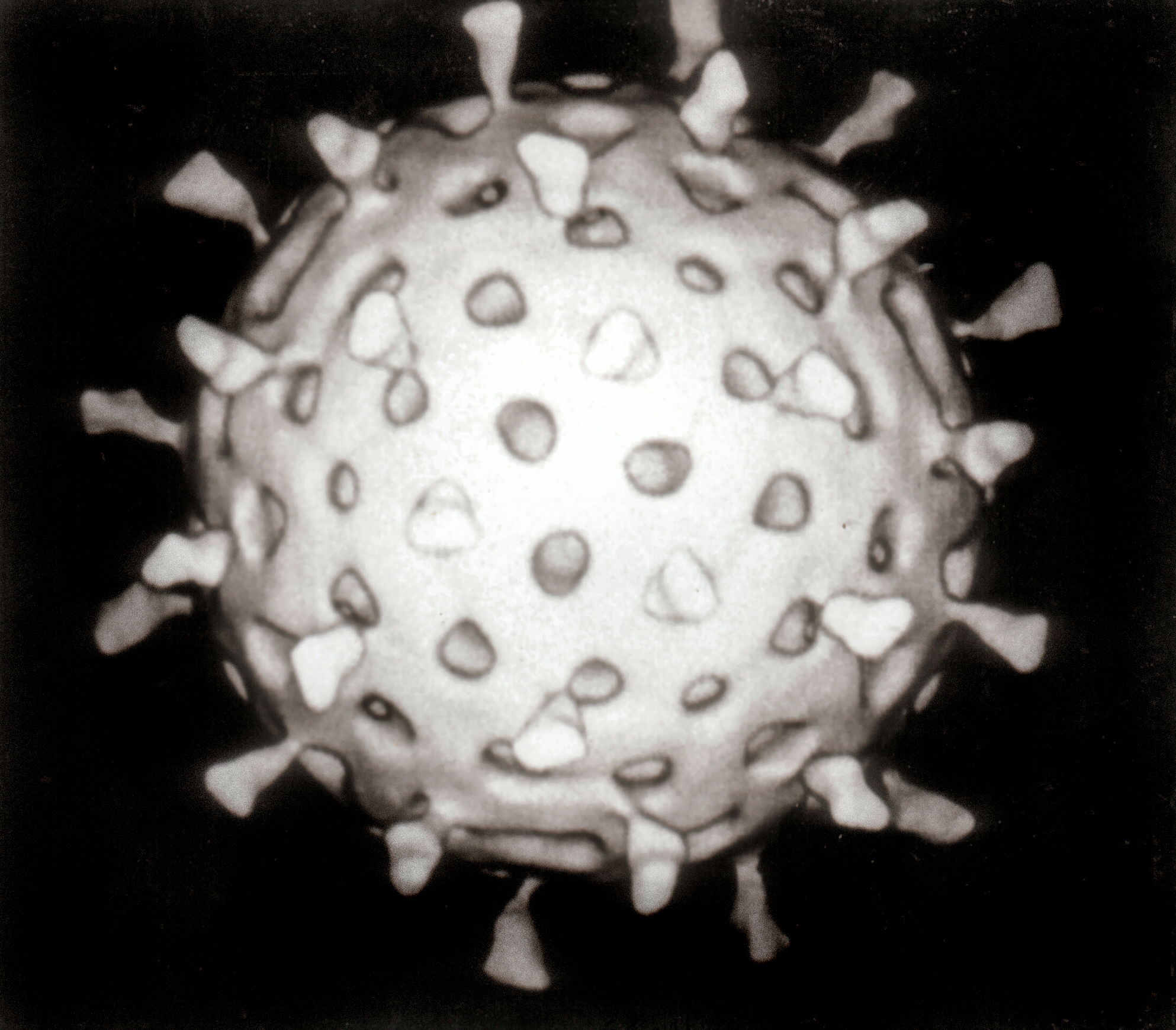
Rotaviruses have a genome consisting of 11 double-stranded RNA segments surrounded by a distinctive three-layered icosahedral protein capsid. The first layer is formed by the protein VP2, with each vertex having a copy of the proteins VP1 and VP3. The second layer is formed by the protein VP6. The outermost protein layer is composed of the structural glycoprotein VP7 and the spike protein VP4. Viral particles are up to 70nm in diameter and have a buoyant density of 1.36 g/ml in CsCl. By negative staining electron microscopy they resemble 'wheels' from which they derive their name (rota is Latin for wheel).
Cell Infection
Rotaviruses tend to affect gastrointestinal epithelial cells that are at the tip of the villus. Their triple protein coats make them very resistant to the normally prohibitive pH of the stomach, and also digestive enzymes (lipases and proteases) in the gastrointestinal tract.
When they infect a cell, they are ingested by the cell in endocytosis in a vesicle known as an endosome. Proteins in the third layer (VP7 and the VP4 spike) disrupt the membrane of the endosome, creating a difference in the Ca2+ concentration. This facilitates the breakdown of VP7 trimers into single protein subunits, leaving the VP2 and VP6 coats around the viral dsRNA, forming a double-layer particle (DLP).
While the eleven dsRNA strands are still within the protection of the two protein shells, RNA-dependent RNA polymerase creates viral mRNA transcripts of the double-stranded viral genome. This is more easily done within the environment in the "core" of the virus than in the host cell's aqueous environment, which significantly slows down the detachment of the two RNA strands to begin mRNA synthesis. Encapsidation of the viral RNA may also serve to evade host immune responses that are triggered by the presence of double-stranded RNA.
During the infection, rotavirus produces mRNA to support both protein translation and genome replication. Most of the rotavirus proteins accumulate in structures known as viroplasms, where the RNA is replicated and the DLPs are assembled. Viroplasms are electron-dense, perinuclear, punctate structures found as early as 2 hours after virus infection. Viroplasms are viral factories and are thought to be formed by two viral non-structural proteins, NSP5 and NSP2. Expression of certain forms of NSP5, especially one that is tagged at the NH2-terminus, results in the formation of viroplasms. Inhibition of NSP5 using intrabodies or RNA interference results in a profound decrease in rotavirus replication. The DLPs can migrate to the endoplasmic reticulum where they obtain their third, outer layer (formed by VP7 and VP4).
Nature of acute disease
Rotaviruses cause acute gastroenteritis. "Infantile diarrhea", "winter diarrhea", "stomach flu", "acute nonbacterial infectious gastroenteritis", and "acute viral gastroenteritis" are other names applied to this disease.
Rotavirus gastroenteritis is a self-limiting, mild to severe disease characterized by vomiting, watery diarrhea, and low-grade fever. The infective dose is presumed to be 10-100 infectious viral particles. Because a person with rotavirus diarrhea often excretes large numbers of virus (108-1010 infectious particles/ml of feces), infection doses can be readily acquired through contaminated hands, objects, or utensils. Asymptomatic rotavirus excretion has been well documented and may play a role in perpetuating endemic disease.
The virus infects enterocytes of the villi of the small intestine, leading to structural changes of the epithelium and diarrhea.
The incubation period ranges from 1-3 days. Symptoms often start with vomiting followed by 4-8 days of diarrhea. Temporary lactose intolerance may occur. Recovery is usually complete. However, severe diarrhea without fluid and electrolyte replacement may result in death. Childhood mortality caused by rotavirus is relatively low in the U.S., with an estimated 100 cases/year, but reaches over 500,000 cases/year worldwide (as of 2005). Association with other enteric pathogens may play a role in the severity of the disease.
Clinically, the most severe disease occurs in children under two years of age (Mandell, Bennett & Dolin: Principles and Practice of Infectious Diseases, 6th ed.)
Target populations and frequency
Humans of all ages are susceptible to rotavirus infection. Children 6 months to 2 years of age, premature infants, the elderly, and the immunocompromised are particularly susceptible to more severe symptoms caused by infection with group A rotavirus.
Group A rotavirus is endemic worldwide. It is the leading cause of severe diarrhea among infants and children, being responsible for about 20% of cases, and accounts for about half of the cases requiring hospitalization. Almost every child has been infected with rotavirus by age 5. Over 3 million cases of rotavirus gastroenteritis occur annually in the U.S. In temperate areas, it occurs primarily in the winter, but in the tropics it occurs throughout the year. The number attributable to food contamination is unknown.
Group B rotavirus, also called adult diarrhea rotavirus or ADRV, has caused major epidemics of severe diarrhea affecting thousands of persons of all ages in China. In a group B epidemic in China in 1982, more than a million people were affected. Group B rotavirus has also been identified after the Chinese epidemics from Calcutta, India in 1998 and this strain was named CAL. Unlike ADRV, the CAL strain is endemic and does not cause known epidemics.
Group C rotavirus has been associated with rare and sporadic cases of diarrhea in children in many countries. However, the first outbreaks were reported from Japan and England.
About 120 million rotavirus infections occur every year, causing the death of 600,000 to 650,000 children.
Diagnosis of human illness
Specific diagnosis of the disease is made by identification of the virus in the patient's stool. Enzyme immunoassay (EIA) is the test most widely used to screen clinical specimens, and several commercial kits are available for group A rotavirus. Electron microscopy and polyacrylamide gel electrophoresis are used in some laboratories in addition or as an alternative to EIA. A reverse transcription-polymerase chain reaction (RT-PCR) has been developed to detect and identify all three groups of human rotaviruses.
Transmission and associated foods
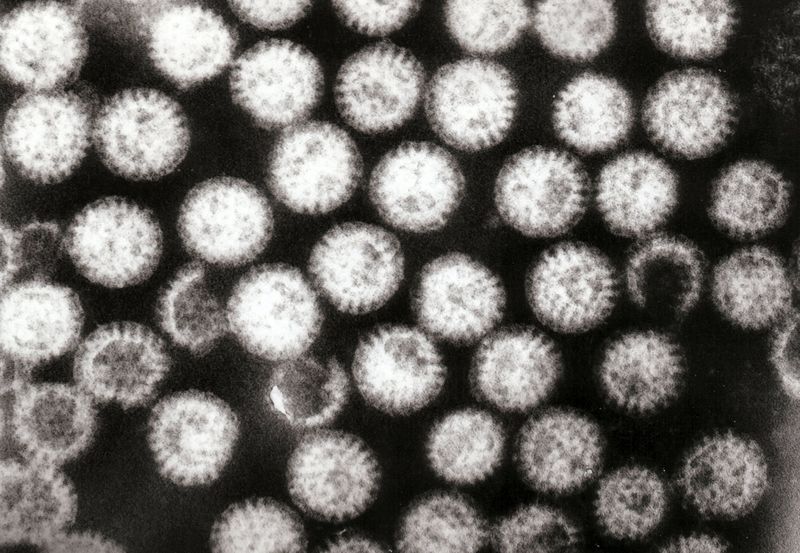
Rotaviruses are transmitted by the fecal-oral route. Person-to-person spread through contaminated hands is probably the most important means by which rotaviruses are transmitted in close communities such as pediatric and geriatric wards, day care centers and family homes.
Infected food handlers may contaminate foods that require handling and no further cooking, such as salads, fruits, and hors d'oeuvres. Rotaviruses are quite stable in the environment and have been found in estuary samples at levels as high as 1-5 infectious particles/gal. Sanitary measures adequate for bacteria and parasites seem to be ineffective in endemic control of rotavirus, as similar incidence of rotavirus infection is observed in countries with both high and low health standards.
The virus has not been isolated from any food associated with an outbreak, and no satisfactory method is available for routine analysis of food. However, it should be possible to apply procedures that have been used to detect the virus in water and in clinical specimens, of which reverse transcription (RT)-PCR amplification is the most sensitive method to food analysis.
Selected outbreaks

Literature references can be found at the links below.
| Country | Rate or range | Published | Source |
|---|---|---|---|
| Vietnam | 1 in 61 to 1 in 113 | 2006 | [1] |
| Bangladesh | 1 in 390 to 1 in 660 | 2007 | [2] |
| Venezuela | 1 in 1800 | 2007 | [3] |
| European Union | 1 in 20433 | 2006 | [4] |
| United States | 1 in 21675 | 2007 | [5] |
Outbreaks of group A rotavirus diarrhea are common among hospitalized infants, young children attending day care centers, and elderly persons in nursing homes. Among adults, multiple foods served in banquets were implicated in 2 outbreaks. An outbreak due to contaminated municipal water occurred in Colorado, 1981.
Several large outbreaks of group B rotavirus involving millions of persons as a result of sewage contamination of drinking water supplies have occurred in China since 1982. Although to date outbreaks caused by group B rotavirus have been confined to mainland China, seroepidemiological surveys have indicated lack of immunity to this group of virus in the U.S. Recent studies led to the identification of group B rotavirus occurring at a sporadic frequency in Calcutta, India and subsequently from other Asian countries as well. Thus, group B rotavirus infection may be more common than presumed earlier, but studies on this pathogen are very limited. Group B rotaviruses are difficult to isolate and cannot be easily adapted to cell culture, a property that precludes their detailed analysis.
The newly recognized group C rotavirus has been implicated in rare and isolated cases of gastroenteritis. However, it was associated with three outbreaks among school children: one in Japan, 1989, and two in England, 1990.
During 2005 the largest recorded outbreak in Nicaragua occurred. This unusual large and severe outbreak was probably due to a mutation in the rotavirus A genome, possibly helping the virus evade the prevalent immunity in the population which had no protection against this type.
For more information on recent outbreaks see the CDC Morbidity and Mortality Weekly Reports.
Complications
Repeated rotavirus infections may increase the risk of celiac disease in genetically susceptible children. A case-control study of infants with a genetic predisposition for celiac disease observed that the risk of developing the disease increased twofold in children who were infected with rotavirus once and almost fourfold for those who were infected with it multiple times (American Journal of Gastroenterology, October 2006.)
Treatment
- Treatment of diarrhoea caused by rotavirus
- Rehydration with oral rehydration salts (ORS) solution. oral rehydration salts (ORS) solution is a mixture of clean water, salt and sugar. It costs a few cents per treatment. oral rehydration salts (ORS) solution is absorbed in the small intestine and replaces the water and electrolytes lost in the faeces.
- Zinc supplements-with zinc supplements reduce the duration of a diarrhoea episode by 25% and are associated with a 30% reduction in stool volume.
- Rehydration with intravenous fluids in case of severe dehydration or shock.
- Nutrient-rich foods the vicious circle of malnutrition and diarrhoea can be broken by continuing to give nutrient-rich foods including breast milk during an episode, and by giving a nutritious diet including exclusive breastfeeding for the first six months of life to children when they are well.
- Consulting a health professional , in particular for management of persistent diarrhoea or when there is blood in stool or if there are signs of dehydration.
- Note (1): Rotavirus and Escherichia coli are the two most common etiological agents of diarrhoea in developing countries.
- Note (2): There is no antiviral drug to treat rotavirus infection. Antibiotic drugs will not help because antibiotics fight against bacteria not viruses.
- Note (3): Rotavirus infection can cause severe vomiting and diarrhea. This can lead to dehydration (loss of body fluids). During rotavirus infection, infants and young children, older adults, and people with other illnesses are most at risk becoming dehydrated.
- Note (4): Symptoms of dehydration include decrease in urination, dry mouth and throat, feeling dizzy when standing up. A dehydrated child may also cry with few or no tears and be unusually sleepy or fussy.
- Prevention
- Access to safe drinking-water
- Use of improved sanitation
- Hand washing with soap
- Exclusive breastfeeding for the first six months of life
- Good personal and food hygiene
- Health education about how infections spread; and Rotavirus vaccination.
Sources
- The Bad Bug Book by the U.S. Food & Drug Administration, 1992.
- New vaccines for diarrhoea virus shown effective, New Scientist, 5 January 2006
- Diarrhoea vaccines prove their mettle, news@nature.com, 5 January 2006
See also
External links
- CDC Viral Gastroenteritis FAQs: Center for Disease Control and Prevention of Food Illness Fact Sheet
- Loci index for genome Rotavirus: Available from the GenBank Taxonomy database, which contains the names of all organisms that are represented in the genetic databases with at least one nucleotide or protein sequence.
- Encyclopedia of Children's Health: Rotavirus Infections: Description, causes and symptoms, diagnosis, treatment, prognosis, prevention, parental concerns, and resources.
- Rotavirus, Emerging Infectious Diseases, Vol 4 No 4, Oct 1998.
- PATH's Rotavirus Vaccine Program
- Rotavirus disease and vaccine resources
ar:فيروس الروتا ca:Rotavirus de:Rotaviren it:Rotavirus lt:Rotavirusinė infekcija nl:Rotavirus fi:Rotavirus
References
- ↑ Anh DD, Thiem VD, Fischer TK, Canh DG, Minh TT, Tho le H, Van Man N, Luan le T, Kilgore P, von Seidlein L, Glass RI (2006). "The burden of rotavirus diarrhea in Khanh Hoa Province, Vietnam: baseline assessment for a rotavirus vaccine trial". Pediatr. Infect. Dis. J. 25 (1): 37–40. PMID 16395100.
- ↑ Tanaka G, Faruque AS, Luby SP, Malek MA, Glass RI, Parashar UD (2007). "Deaths from rotavirus disease in Bangladeshi children: estimates from hospital-based surveillance". Pediatr. Infect. Dis. J. 26 (11): 1014–8. doi:10.1097/INF.0b013e318125721c. PMID 17984808.
- ↑ Pérez-Schael I, Salinas B, González R, Salas H, Ludert JE, Escalona M, Alcalá A, Rosas MA, Materán M (2007). "Rotavirus mortality confirmed by etiologic identification in Venezuelan children with diarrhea". Pediatr. Infect. Dis. J. 26 (5): 393–7. doi:10.1097/01.inf.0000260252.48129.86. PMID 17468648.
- ↑ Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T (2006). "Burden of rotavirus disease in European Union countries". Pediatr. Infect. Dis. J. 25 (1 Suppl): S7–S11. PMID 16397431.
- ↑
- ↑ Template:Citeweb
- ↑ Template:Citeweb