Reperfusion injury pathophysiology: Difference between revisions
Sara Mohsin (talk | contribs) No edit summary |
Sara Mohsin (talk | contribs) No edit summary |
||
| Line 2: | Line 2: | ||
{{Reperfusion injury}} | {{Reperfusion injury}} | ||
{{CMG}} {{AE}} {{AC}} {{Shivam Singla}} [[User:Kashish Goel|Kashish Goel,M.D.,]] | |||
==Overview== | ==Overview== | ||
Revision as of 19:30, 20 August 2020
|
Reperfusion injury Microchapters |
|
Treatment |
|---|
|
Reperfusion injury pathophysiology On the Web |
|
American Roentgen Ray Society Images of Reperfusion injury pathophysiology |
|
Risk calculators and risk factors for Reperfusion injury pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Anjan K. Chakrabarti, M.D. [2] Shivam Singla, M.D.[3] Kashish Goel,M.D.,
Overview
Pathophysiology
Mainly divided into 2 phases
1) Ischemic phase
2) Reperfusion Phase
Ischemic Phase
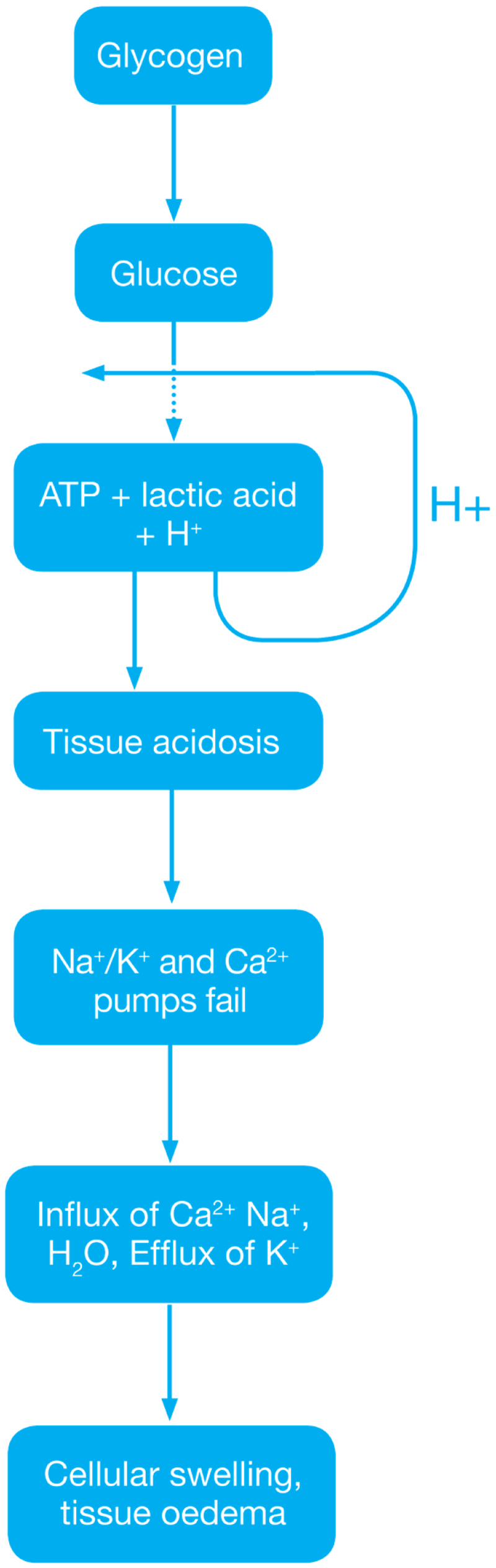
Reperfusion injury ( Ischemic Phase) During this phase mainly the dysregulation of metabolic pathways occurs and in the reperfusion phase there will be generation of free radicals.
- Ischemia when the blood supply to the tissues decreases with respect to the demand required to function properly. This results in deficiency in oxygen, glucose and various other substrates required for cellular metabolism. As previously dais the derangement or dysregulation of metabolic function begins in this phase. Due to less oxygen supply cellular metabolism shifts to anaerobic glycolysis causing the glycogen to breakdown resulting in the production of 2 ATP and a lactic acid. This decrease in tissue PH starts further inhibits the ATP generation by negative feed back mechanism. ATP gets broken down into ADP, AMP and IMP. This finally gets converted to adenosine, inosine, hypoxanthine and xanthine.
- Lack of ATP at the cellular level causes impairment in the function of ionic pumps - Na+/K+ and Ca2+ pumps. As a result cytosolic sodium rises which in turn withdraws water to maintain the osmotic equilibrium consequently resulting in the cellular swelling. To maintain ionic balance potassium ion escape from the cell. Calcium is released from the mitochondria to the cytoplasm and into extracellular spaces resulting in the activation of Mitochondrial calcium- dependent cytosolic proteases. These converts the enzyme xanthine dehydrogenase to xanthine oxidase. Phospholipases activated during ischemia promotes membrane degradation and increases level of free fatty acids
- Ischemia also induces expression of a large number of genes and transcription factors, which play a major role in the damage to the tissues.
- Transcription factors
- Activating protein-1 (AP-1)
- Hypoxia-inducible factor-1 (HIF-1) which in turn activates transcription of VEGF, Erythropoietin and Glucose transporter-1
- Nuclear factor-kappa b (NF-kb)
- Activation of NF-kb occurs during both the ischemic and reperfusion phases
- Transcription factors
Reperfusion Phase
Reactive oxygen species
The ROS play major role in the tissue damage related to ischemia reperfusion injury. Once the ischemic tissue is reperfused the molecular oxygen catalyzes the conversion of hypoxanthine to uric acid and liberating the superoxide anion (O2-). This superoxide gets further converted to (H2O2) and the hydroxyl radical (OH•). This OH ion causes the peroxidation lipids in the cell membranes resulting in the production and release of proinflammatory eicosanoids and ultimately cell death. Reperfusion Injury

During the Ischemia reperfusion injury ROS also activate endothelial cells, which further produces numerous adhesion molecules.
- E-selectin
- VCAM-1 (vascular cell adhesion molecule-1)
- ICAM-1 (intercellular adhesion molecule-1)
- EMLMl Am -1 ( endothelial-leukocyte adhesion molecule)
- PAi-1 (plasminogen activator inhibitor-1 ), and
- Interleukin-8 (il-8)
Eicosanoids
ROS causes lipid peroxidation of cell membranes resulting in release of
- Arachidonic acid (substrate for prostaglandins)
- Prostaglandins usually have a vasodilatory effect hat provides protective effect during Ischemia reperfusion injury. But they have short life so their fast depletion leads to vasoconstriction ultimately leading to reduced blood flow and exacerbation of ischemia.
- Thromboxane
- Plasma thromboxane A2 level rises within minutes after reperfusion, resulting in vasoconstriction and platelet aggregation. This usually coincide with rapid rise in pulmonary artery pressure and a subsequent increase in pulmonary microvascular permeability.
- Leukotrienes
- Leukotrienes are also synthesized from arachidonic acid. Leukotrienes acts directly in the endothelial cells, smooth muscle and indirectly on the neutrophils. The leukotrienes C4, D4, and E4 alters the endothelial cytoskeleton, resulting in increased vascular permeability and smooth muscle contraction, and finally leading to vasoconstriction.
Nitric oxide
L-arginine is the substrate for the synthesis of Nitric oxide with the help of nitric oxide synthase enzyme. The nitric oxide synthase enzyme is usually of 3 types
- CNOS- Constitutive nitric oxide synthase enzyme
- INO S- Inducible nitric oxide synthase enzyme
- ENO S- Endothelial nitric oxide synthase enzyme
In the first 15 minutes of ischemia NO level rises due to transient ENOS activation. As said this elevation is transient so ultimately after few minutes there will be general decline in endothelial function resulting in fall of NO production. The reduction in ENOS levels during ischemia reperfusion injury are also predispose to vasoconstriction , the response mainly seen in IRI.
Endothelin
These are peptide vasoconstrictors mainly produced from the endothelium. They mainly mediate vasoconstriction through Ca2+-mediated vasoconstriction. Endothelin -1 levels increase during ischemia reperfusion injury in both the phases of ischemia as well as reperfusion, that mainly help in capillary vasoconstriction. Endothelin - 1 inhibitors are studied widespread regarding their role in inhibiting vasoconstriction and increasing vascular permeability.
Cytokines
Ischemia and reperfusion phase of ischemia reperfusion injury induces expression of numerous cytokines mainly:
- TNF-a
- Elevated levels detected during cerebral and skeletal IRI. it can also induce generation of ROS and enhance the susceptibility of vascular endothelium to neutrophil mediated injury by increasing the expression of ICAM-1 which helps in binding of neutrophils to the endothelium.
- IL-1, IL-6, IL-8
- IL-6 is a proinflammatory cytokine produces in large amounts in hypo perfused tissues.
- IL-8 is a neutrophil chemotactic and activating factor and mainly results in the diapedesis of activated neutrophils through the endothelium.
- PAF
- It enhances the binding of neutrophils to the endothelial cells.
These cytokines mainly generate systemic inflammatory response ultimately leads to multi organ failure.
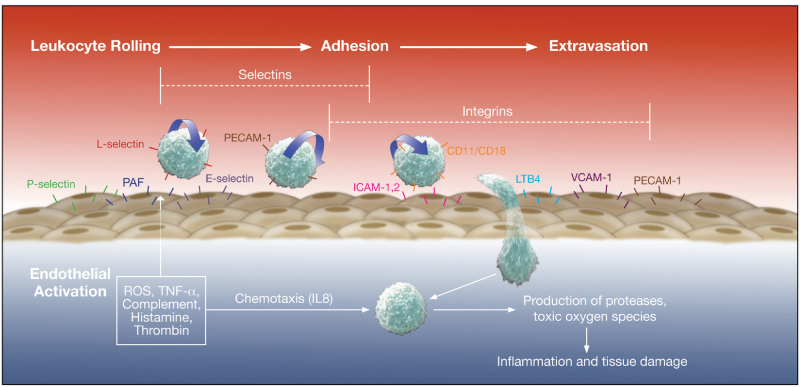
Neutrophils and endothelial interactions
Neutrophils plays Important role in the tissue damage. Activated neutrophils secrete proteases, metalloproteinase, that results in the degradation of basement membrane and contributes to tissue damage. Selectins are expressed on the surface of leucocytes, endothelial cells and platelets. Selectins play important role in the initiation of neutrophil–endothelial cell interactions (rolling) which is essential for their subsequent adhesion and extravasation. L-selectin are present on surface of neutrophils and help in the reversible attachment of neutrophils to endothelial cells. Antibody-mediated blocking of L-selectin studied widely and is one of the important treatment option under consideration.
Complement activation
Contributes in the pathogenesis of IRI. Reperfusion is usually associated with depletion of complement proteins, factor B that will indicates the turning on of alternate complement pathway. The C5b-9 also gets deposited into the endothelial cell after ischemia leading to osmotic lysis.
Main Central organs affected in reperfusion injury.
Central Nervous System
Reperfusion injury is a major pathophysiological mechanism involved in ischemia related injury to the central nervous system consequently resulting the patients to land up with complications of stoke , TIA and other neurological problems. A lot of studies regarding this are still under pipe line.
Cardiovascular system
In cardiovascular system the most common complications studied are arrhythmias, and myocardial stunning and myocardial cell also. According to various studies done so far, Impaired microvascular function is the main reason behind the myocardial stunning