Reperfusion injury pathophysiology: Difference between revisions
| Line 3: | Line 3: | ||
'''Editors-In-Chief:''' {{AC}}; [[C. Michael Gibson]], M.S., M.D. [mailto:Mgibson@perfuse.org]; [[User:Shivam Singla|Dr. Shivam Singla M.D [1]]], [[User:Kashish Goel|Kashish Goel,M.D.,]] | '''Editors-In-Chief:''' {{AC}}; [[C. Michael Gibson]], M.S., M.D. [mailto:Mgibson@perfuse.org]; [[User:Shivam Singla|Dr. Shivam Singla M.D [1]]], [[User:Kashish Goel|Kashish Goel,M.D.,]] | ||
== | ==Pathophysiology == | ||
[[ | === Mainly divided into 2 phases === | ||
1) [[Ischemia|Ischemi]]<nowiki/>c phase | |||
2) [[Reperfusion|Reperfusio]]<nowiki/>n Phase | |||
[[File:Reperfusion Injury ( Ischemic Phase).jpg|thumb|515x515px|Reperfusion Injury (Ischemic phase)]] | |||
=== Ischemic Phase === | |||
Reperfusion injury ( Ischemic Phase) | |||
During this phase mainly the dysregulation of [[Metabolic pathway|metabolic pathways]] occurs and in the [[Reperfusion|reperfusion phase]] there will be generation of [[free radicals]]. | |||
* [[Ischemia]] when the [[blood]] supply to the [[Tissue (biology)|tissues]] decreases with respect to the demand required to function properly. This results in [[deficiency]] in [[oxygen]], [[glucose]] and various other substrates required for [[cellular metabolism]]. As previously dais the derangement or dysregulation of metabolic function begins in this phase. Due to less [[oxygen]] supply [[cellular metabolism]] shifts to [[anaerobic]] [[glycolysis]] causing the [[glycogen]] to breakdown resulting in the production of 2 ATP and a [[lactic acid]]. This decrease in tissue PH starts further inhibits the [[Adenosine triphosphate|ATP generation]] by negative feed back mechanism. [[Adenosine triphosphate|ATP]] gets broken down into [[Adenosine diphosphate|ADP]], [[Adenosine monophosphate|AMP]] and [[Inosine monophosphate|IMP]]. This finally gets converted to [[adenosine]], [[inosine]], [[hypoxanthine]] and [[xanthine]]. | |||
* Lack of [[Adenosine triphosphate|ATP]] at the cellular level causes impairment in the function of ionic pumps - [[Na+/K+-ATPase|Na+/K+]] and Ca<sup>2</sup>+ pumps. As a result [[cytosolic]] sodium rises which in turn withdraws water to maintain the [[Osmosis|osmotic]] [[equilibrium]] consequently resulting in the [[cellular]] [[Swelling (medical)|swelling]]. To maintain ionic balance [[Potassium ion channels|potassium ion]] escape from the cell. [[Calcium]] is released from the [[Mitochondrion|mitochondria]] to the cytoplasm and into extracellular spaces resulting in the activation of Mitochondrial calcium- dependent [[Proteases|cytosolic proteases]]. These converts the enzyme [[xanthine dehydrogenase]] to [[xanthine oxidase]]. Phospholipases activated during [[ischemia]] promotes membrane degradation and increases level of [[Fatty acid|free fatty acids]] | |||
* [[Ischemia]] also induces expression of a large number of [[genes]] and [[Transcription factor|transcription factors]], which play a major role in the damage to the tissues. | |||
** Transcription factors | |||
*** Activating protein-1 ([[AP-1 (transcription factor)|AP-1]]) | |||
*** Hypoxia-inducible factor-1 (HIF-1) which in turn activates transcription of VEGF, [[Erythropoietin]] and [[Glucose transporter|Glucose transporter-1]] | |||
*** Nuclear factor-kappa b ([[NF-kB|NF-kb]]) | |||
*** Activation of NF-kb occurs during both the [[Ischemia|ischemic]] and [[reperfusion]] phases | |||
<br /> | |||
=== Reperfusion Phase === | |||
==== Reactive oxygen species ==== | |||
The ROS play major role in the tissue damage related to [[ischemia]] [[reperfusion injury]]. Once the ischemic tissue is reperfused the molecular [[oxygen]] catalyzes the conversion of [[hypoxanthine]] to [[uric acid]] and liberating the [[Superoxide|superoxide anion]] (O<sub>2</sub><sup>-</sup>). This superoxide gets further converted to (H<sub>2</sub>O<sub>2</sub>) and the [[hydroxyl radical]] ([[Hydroxyl radical|OH<sup>•</sup>)]]. This OH ion causes the peroxidation [[Lipid|lipids]] in the [[Cell membrane|cell membranes]] resulting in the production and release of proinflammatory [[Eicosanoid|eicosanoids]] and ultimately cell death. | |||
Reperfusion Injury | |||
[[File:Reperfusion Injury Mech.jpg|thumb|Reperfusion injury ( Reperfusion phase)]] | |||
During the Ischemia reperfusion injury ROS also activate [[Endothelium|endothelial cells]], which further produces numerous [[Cell adhesion molecule|adhesion molecules.]] | |||
* [[E-selectin]] | |||
* [[VCAM-1]] (vascular cell adhesion molecule-1) | |||
* [[ICAM-1]] (intercellular adhesion molecule-1) | |||
* EMLMl Am -1 ( endothelial-leukocyte adhesion molecule) | |||
* [[Plasminogen activator inhibitor-1|PAi-1]] (plasminogen activator inhibitor-1 ), and | |||
* [[Interleukin 8|Interleukin-8]] (il-8) | |||
==== Eicosanoids ==== | |||
ROS causes [[lipid peroxidation]] of cell membranes resulting in release of | |||
* ''[[Arachidonic acid]] (substrate for [[Prostaglandin|prostaglandins]])'' | |||
** Prostaglandins usually have a [[Vasodilatory|vasodilatory effect]] hat provides protective effect during [[Ischemia]] reperfusion injury. But they have short life so their fast depletion leads to [[vasoconstriction]] ultimately leading to reduced blood flow and exacerbation of [[ischemia]]. | |||
* ''[[Thromboxane]]'' | |||
** [[Thromboxane A2|Plasma thromboxane A<sub>2</sub>]] level rises within minutes after reperfusion, resulting in [[vasoconstriction]] and [[platelet aggregation]]. This usually coincide with rapid rise in [[Pulmonary artery hypertension|pulmonary artery pressure]] and a subsequent increase in [[Lung|pulmonary]] [[Microvascular bed|microvascular]] permeability. | |||
* ''[[Leukotriene|Leukotrienes]]'' | |||
** Leukotrienes are also synthesized from arachidonic acid. [[Leukotriene]]<nowiki/>s acts directly in the [[endothelial cells]], [[smooth muscle]] and indirectly on the [[neutrophils]]. The [[Leukotriene|leukotrienes]] C<sub>4</sub>, D<sub>4,</sub> and E<sub>4</sub> alters the endothelial [[cytoskeleton]], resulting in increased [[vascular]] permeability and [[smooth muscle]] contraction, and finally leading to [[vasoconstriction]]. | |||
==== Nitric oxide ==== | |||
[[L-arginine]] is the substrate for the synthesis of [[Nitric oxide]] with the help of nitric oxide synthase enzyme. The [[nitric oxide synthase]] enzyme is usually of 3 types | |||
* CNOS- Constitutive [[Nitric oxide synthase|nitric oxide synthase enzyme]] | |||
* INO S- Inducible [[Nitric oxide synthase|nitric oxide synthase enzyme]] | |||
* ENO S- [[Endothelial|Endothelia]]<nowiki/>l nitric oxide synthase enzyme | |||
In the first 15 minutes of ischemia [[Nitric oxide|NO]] level rises due to transient ENOS activation. As said this elevation is transient so ultimately after few minutes there will be general decline in [[Endothelium|endothelial function]] resulting in fall of NO production. The reduction in ENOS levels during ischemia reperfusion injury are also predispose to vasoconstriction , the response mainly seen in IRI. | |||
[[File:Neutrophils involved in tissue destruction.jpg|thumb|329x329px|Neutrophils, attachment, rolling and extravasation]] | |||
==== Endothelin ==== | |||
These are peptide [[Vasoconstrictor|vasoconstrictors]] mainly produced from the [[endothelium]]. They mainly mediate [[vasoconstriction]] through Ca<sup>2+</sup>-mediated vasoconstriction. [[Endothelin-1|Endothelin -1]] levels increase during [[Ischemia-reperfusion injury|ischemia reperfusion injury]] in both the phases of [[ischemia]] as well as [[reperfusion]], that mainly help in [[capillary]] vasoconstriction. Endothelin - 1 inhibitors are studied widespread regarding their role in inhibiting [[vasoconstriction]] and increasing [[vascular permeability]]. | |||
==== Cytokines ==== | |||
[[Ischemia]] and reperfusion phase of [[ischemia]] [[reperfusion injury]] induces expression of numerous [[Cytokine|cytokines]] mainly: | |||
* [[Tumor necrosis factor-alpha|TNF-a]] | |||
** Elevated levels detected during [[cerebral]] and [[skeletal]] IRI. it can also induce generation of ROS and enhance the susceptibility of vascular [[endothelium]] to neutrophil mediated injury by increasing the expression of [[ICAM-1]] which helps in binding of [[Neutrophil|neutrophils]] to the [[endothelium]]. | |||
* [[IL-1|IL-1, IL-6, IL-8]] | |||
** IL-6 is a proinflammatory [[cytokine]] produces in large amounts in hypo perfused [[Tissue (biology)|tissues]]. | |||
** IL-8 is a [[neutrophil]] [[Chemotaxis|chemotactic]] and activating factor and mainly results in the [[diapedesis]] of activated [[Neutrophil|neutrophils]] through the [[endothelium]]. | |||
* PAF | |||
** It enhances the binding of [[Neutrophil|neutrophils]] to the [[endothelial cells]]. | |||
These [[Cytokine|cytokines]] mainly generate systemic inflammatory response ultimately leads to multi [[organ failure]]. | |||
==== Neutrophils and endothelial interactions ==== | |||
[[Neutrophil|Neutrophils]] plays Important role in the tissue damage. Activated neutrophils secrete [[Protease|proteases]], [[metalloproteinase]], that results in the degradation of [[basement membrane]] and contributes to tissue damage. [[Selectin|Selectins]] are expressed on the surface of [[leucocytes]], [[endothelial cells]] and [[platelets]]. Selectins play important role in the initiation of neutrophil–endothelial cell interactions (rolling) which is essential for their subsequent [[adhesion]] and [[extravasation]]. [[L-selectin]] are present on surface of [[Neutrophil|neutrophils]] and help in the reversible attachment of neutrophils to [[endothelial cells]]. Antibody-mediated blocking of L-selectin studied widely and is one of the important treatment option under consideration. | |||
==== Complement activation ==== | |||
Contributes in the pathogenesis of IRI. [[Reperfusion]] is usually associated with depletion of [[Complement|complement proteins]], [[factor B]] that will indicates the turning on of alternate complement pathway. The C5b-9 also gets deposited into the endothelial cell after [[ischemia]] leading to [[Osmotic lysis|osmotic lysis.]] | |||
<br /> | |||
===Specific organs affected by reperfusion injury=== | |||
==== CNS ==== | |||
Reperfusion injury plays a part in the [[brain]]'s [[ischemic cascade]], which is involved in [[stroke]] and [[brain trauma]]. Repeated bouts of ischemia and reperfusion injury also are thought to be a factor leading to the formation and failure to [[wound healing|heal]] of [[chronic wound]]s such as [[pressure sore]]s and [[diabetic foot]] [[ulcer]]s. Continuous pressure limits blood supply and causes ischemia, and the [[inflammation]] occurs during reperfusion. As this process is repeated, it eventually damages tissue enough to cause a [[wound]] | |||
==== CVS ( Myocardium) ==== | |||
Restoration of [[epicardial]] patency can be associated with r[[Reperfusion injury|eperfusion injury]] in the myocardium. This can manifest clinically as [[Cardiac arrhythmia|arrhythmia]], microvascular dysfunction, [[Stunned myocardium|myocardial stunning]], and myocyte death. | |||
Microvascular dysfunction, or "no reflow," as well as myocardial stunning, are the possible consequences of [[reperfusion injury]]. Myocardial stunning, may to some extent be mediated by impaired [[Microvascular bed|microvascular function]]. | |||
==References== | ==References== | ||
[[Category:Physiology]] | [[Category:Physiology]] | ||
[[Category:Neurotrauma]] | [[Category:Neurotrauma]] | ||
| Line 38: | Line 102: | ||
[[Category:Up-To-Date]] | [[Category:Up-To-Date]] | ||
[[Category:Up-To-Date cardiology]] | [[Category:Up-To-Date cardiology]] | ||
Revision as of 23:55, 11 August 2020
|
Reperfusion injury Microchapters |
|
Treatment |
|---|
|
Reperfusion injury pathophysiology On the Web |
|
American Roentgen Ray Society Images of Reperfusion injury pathophysiology |
|
Risk calculators and risk factors for Reperfusion injury pathophysiology |
Editors-In-Chief: Anjan K. Chakrabarti, M.D. [1]; C. Michael Gibson, M.S., M.D. [2]; Dr. Shivam Singla M.D [1], Kashish Goel,M.D.,
Pathophysiology
Mainly divided into 2 phases
1) Ischemic phase
2) Reperfusion Phase
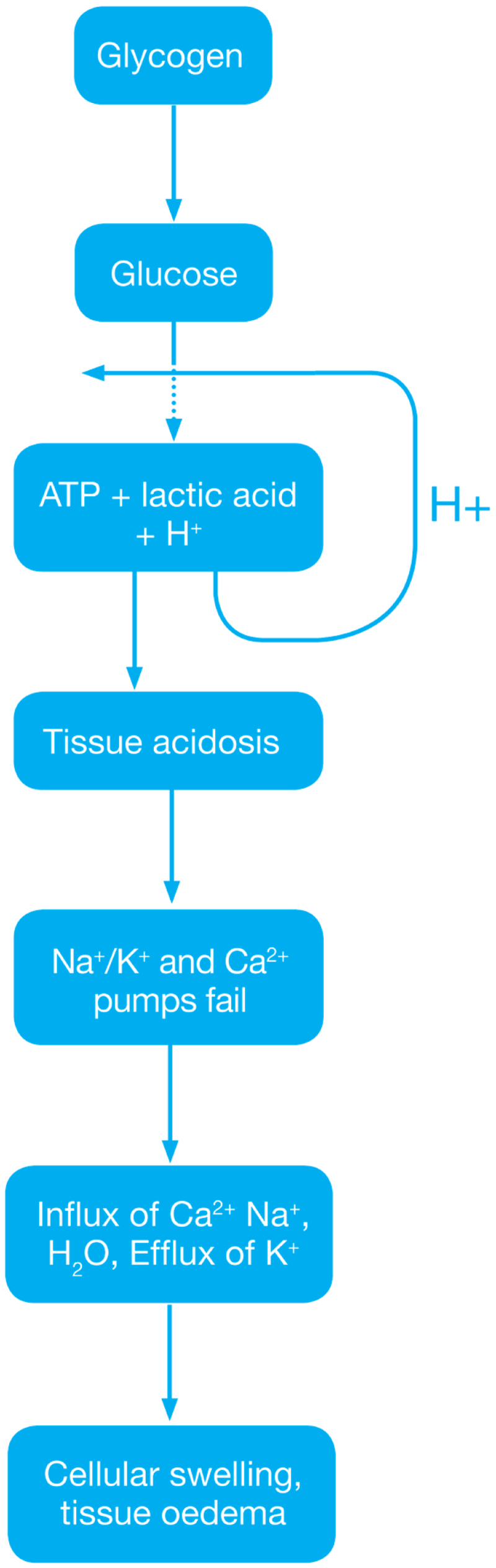
Ischemic Phase
Reperfusion injury ( Ischemic Phase) During this phase mainly the dysregulation of metabolic pathways occurs and in the reperfusion phase there will be generation of free radicals.
- Ischemia when the blood supply to the tissues decreases with respect to the demand required to function properly. This results in deficiency in oxygen, glucose and various other substrates required for cellular metabolism. As previously dais the derangement or dysregulation of metabolic function begins in this phase. Due to less oxygen supply cellular metabolism shifts to anaerobic glycolysis causing the glycogen to breakdown resulting in the production of 2 ATP and a lactic acid. This decrease in tissue PH starts further inhibits the ATP generation by negative feed back mechanism. ATP gets broken down into ADP, AMP and IMP. This finally gets converted to adenosine, inosine, hypoxanthine and xanthine.
- Lack of ATP at the cellular level causes impairment in the function of ionic pumps - Na+/K+ and Ca2+ pumps. As a result cytosolic sodium rises which in turn withdraws water to maintain the osmotic equilibrium consequently resulting in the cellular swelling. To maintain ionic balance potassium ion escape from the cell. Calcium is released from the mitochondria to the cytoplasm and into extracellular spaces resulting in the activation of Mitochondrial calcium- dependent cytosolic proteases. These converts the enzyme xanthine dehydrogenase to xanthine oxidase. Phospholipases activated during ischemia promotes membrane degradation and increases level of free fatty acids
- Ischemia also induces expression of a large number of genes and transcription factors, which play a major role in the damage to the tissues.
- Transcription factors
- Activating protein-1 (AP-1)
- Hypoxia-inducible factor-1 (HIF-1) which in turn activates transcription of VEGF, Erythropoietin and Glucose transporter-1
- Nuclear factor-kappa b (NF-kb)
- Activation of NF-kb occurs during both the ischemic and reperfusion phases
- Transcription factors
Reperfusion Phase
Reactive oxygen species
The ROS play major role in the tissue damage related to ischemia reperfusion injury. Once the ischemic tissue is reperfused the molecular oxygen catalyzes the conversion of hypoxanthine to uric acid and liberating the superoxide anion (O2-). This superoxide gets further converted to (H2O2) and the hydroxyl radical (OH•). This OH ion causes the peroxidation lipids in the cell membranes resulting in the production and release of proinflammatory eicosanoids and ultimately cell death. Reperfusion Injury

During the Ischemia reperfusion injury ROS also activate endothelial cells, which further produces numerous adhesion molecules.
- E-selectin
- VCAM-1 (vascular cell adhesion molecule-1)
- ICAM-1 (intercellular adhesion molecule-1)
- EMLMl Am -1 ( endothelial-leukocyte adhesion molecule)
- PAi-1 (plasminogen activator inhibitor-1 ), and
- Interleukin-8 (il-8)
Eicosanoids
ROS causes lipid peroxidation of cell membranes resulting in release of
- Arachidonic acid (substrate for prostaglandins)
- Prostaglandins usually have a vasodilatory effect hat provides protective effect during Ischemia reperfusion injury. But they have short life so their fast depletion leads to vasoconstriction ultimately leading to reduced blood flow and exacerbation of ischemia.
- Thromboxane
- Plasma thromboxane A2 level rises within minutes after reperfusion, resulting in vasoconstriction and platelet aggregation. This usually coincide with rapid rise in pulmonary artery pressure and a subsequent increase in pulmonary microvascular permeability.
- Leukotrienes
- Leukotrienes are also synthesized from arachidonic acid. Leukotrienes acts directly in the endothelial cells, smooth muscle and indirectly on the neutrophils. The leukotrienes C4, D4, and E4 alters the endothelial cytoskeleton, resulting in increased vascular permeability and smooth muscle contraction, and finally leading to vasoconstriction.
Nitric oxide
L-arginine is the substrate for the synthesis of Nitric oxide with the help of nitric oxide synthase enzyme. The nitric oxide synthase enzyme is usually of 3 types
- CNOS- Constitutive nitric oxide synthase enzyme
- INO S- Inducible nitric oxide synthase enzyme
- ENO S- Endothelial nitric oxide synthase enzyme
In the first 15 minutes of ischemia NO level rises due to transient ENOS activation. As said this elevation is transient so ultimately after few minutes there will be general decline in endothelial function resulting in fall of NO production. The reduction in ENOS levels during ischemia reperfusion injury are also predispose to vasoconstriction , the response mainly seen in IRI.
Endothelin
These are peptide vasoconstrictors mainly produced from the endothelium. They mainly mediate vasoconstriction through Ca2+-mediated vasoconstriction. Endothelin -1 levels increase during ischemia reperfusion injury in both the phases of ischemia as well as reperfusion, that mainly help in capillary vasoconstriction. Endothelin - 1 inhibitors are studied widespread regarding their role in inhibiting vasoconstriction and increasing vascular permeability.
Cytokines
Ischemia and reperfusion phase of ischemia reperfusion injury induces expression of numerous cytokines mainly:
- TNF-a
- Elevated levels detected during cerebral and skeletal IRI. it can also induce generation of ROS and enhance the susceptibility of vascular endothelium to neutrophil mediated injury by increasing the expression of ICAM-1 which helps in binding of neutrophils to the endothelium.
- IL-1, IL-6, IL-8
- IL-6 is a proinflammatory cytokine produces in large amounts in hypo perfused tissues.
- IL-8 is a neutrophil chemotactic and activating factor and mainly results in the diapedesis of activated neutrophils through the endothelium.
- PAF
- It enhances the binding of neutrophils to the endothelial cells.
These cytokines mainly generate systemic inflammatory response ultimately leads to multi organ failure.
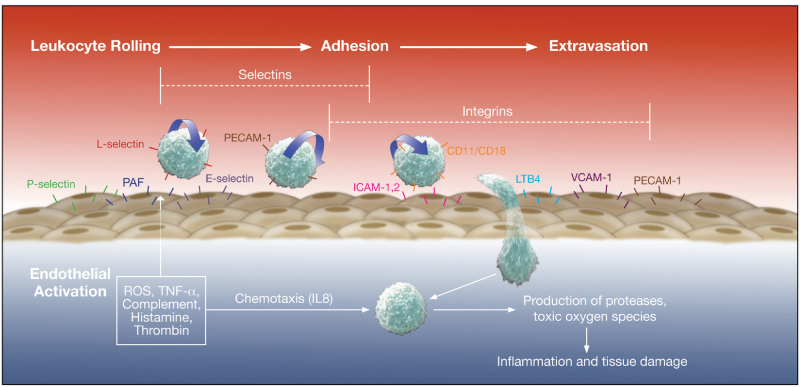
Neutrophils and endothelial interactions
Neutrophils plays Important role in the tissue damage. Activated neutrophils secrete proteases, metalloproteinase, that results in the degradation of basement membrane and contributes to tissue damage. Selectins are expressed on the surface of leucocytes, endothelial cells and platelets. Selectins play important role in the initiation of neutrophil–endothelial cell interactions (rolling) which is essential for their subsequent adhesion and extravasation. L-selectin are present on surface of neutrophils and help in the reversible attachment of neutrophils to endothelial cells. Antibody-mediated blocking of L-selectin studied widely and is one of the important treatment option under consideration.
Complement activation
Contributes in the pathogenesis of IRI. Reperfusion is usually associated with depletion of complement proteins, factor B that will indicates the turning on of alternate complement pathway. The C5b-9 also gets deposited into the endothelial cell after ischemia leading to osmotic lysis.
Specific organs affected by reperfusion injury
CNS
Reperfusion injury plays a part in the brain's ischemic cascade, which is involved in stroke and brain trauma. Repeated bouts of ischemia and reperfusion injury also are thought to be a factor leading to the formation and failure to heal of chronic wounds such as pressure sores and diabetic foot ulcers. Continuous pressure limits blood supply and causes ischemia, and the inflammation occurs during reperfusion. As this process is repeated, it eventually damages tissue enough to cause a wound
CVS ( Myocardium)
Restoration of epicardial patency can be associated with reperfusion injury in the myocardium. This can manifest clinically as arrhythmia, microvascular dysfunction, myocardial stunning, and myocyte death.
Microvascular dysfunction, or "no reflow," as well as myocardial stunning, are the possible consequences of reperfusion injury. Myocardial stunning, may to some extent be mediated by impaired microvascular function.