Reperfusion injury overview: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
Pathophysiological Mechanism is as follows: | Pathophysiological Mechanism is as follows: | ||
* The pathophysiologic mechanisms underlying reperfusion injury | * The pathophysiologic mechanisms underlying reperfusion injury includes various steps starting from infarction, inflammation, generation of free radicals, an increase in intracellular calcium, development of edema, mitochondrial damage and finally leading to activation of coagulation. | ||
* '''Reperfusion | *'''[[Reperfusion]]''' damage occurs after myocardial reperfusion after a period of decreased [[oxygen]] supply. The damage from reperfusion injury is partially due to the affected tissue's inflammatory response. [[White blood cells]] transported to the region by fresh blood release a host of inflammatory factors such as [[Interleukin|interleukins]] and free radicals in response to tissue injury. Blood flow restored reintroduces oxygen inside cells that damages cellular proteins, DNA, and plasma membrane. Damage to the membrane of the cell will in effect cause further free radicals to be released. These reactive species can also act indirectly to turn on apoptosis through redox signaling. Also, leukocytes may build up in small capillaries, block them and cause more ischemia | ||
[[File:Mechanism of Reperfusion injury.jpg|border|397x397px|Mechanism Of Reperfusion injury|right]] | [[File:Mechanism of Reperfusion injury.jpg|border|397x397px|Mechanism Of Reperfusion injury|right]] | ||
* Mitochondrial dysfunction plays | * Mitochondrial dysfunction plays a significant role in reperfusion injury. Although the mitochondrial membrane is normally impermeable to ions and metabolites, ischemia changes permeability by elevating concentrations of intro-mitochondrial calcium, reducing concentrations of adenine nucleotides, and inducing oxidative stress. It gives primacy to the mitochondrial transfer pore permeability (MPTP), which opens when reperfusion occurs. This contributes to increased osmotic load in the mitochondrial body causing swelling and breakup, releasing proteins that induce apoptosis from mitochondria. Mitochondrial activity is impaired, and ATP is hydrolyzed, allowing degrading enzymes to activate. Finally, excessive activation of the Poly ADP ribose polymerase-1 (PARP-1) impairs the work of other organelles and speeds up the development of reactive oxygen species. | ||
* | * Hypoxanthine is produced as breakdown product of the ATP metabolism in prolonged ischemia (60 minutes or more). As a consequence of higher oxygen availability the enzyme xanthine dehydrogenase is converted to xanthine oxidase. This oxidation contributes to the conversion of molecular oxygen into highly reactive superoxide and hydroxyl radicals. Xanthine oxidase also produces uric acid, which can act both as a pro-oxidant and as a reactive species scavenger such as per-oxinitrite. To produce the potent reactive species per-oxinitrite, too much nitric oxide formed during reperfusion reacts with superoxide. These radicals and reactive oxygen species attack lipids , proteins, and glycosaminoglycan from the cell membrane, causing further damage. Specific biological processes may also be initiated by redox signaling. | ||
<br /> | |||
==Risk Factors== | ==Risk Factors== | ||
Risk factors for reperfusion injury include | Risk factors for reperfusion injury include | ||
* [[Hypertension]] with [[left ventricular hypertrophy]], | |||
* [[Congestive heart failure]], | |||
* Increased age, | |||
* [[Diabetes]], and | |||
* [[Hyperlipidemia]] | |||
==Natural History, Complications and Prognosis== | ==Natural History, Complications and Prognosis== | ||
Revision as of 20:48, 30 July 2020
Editors-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editors-In-Chief: Shivam Singla, M.D
Overview

The introduction and wide application of reperfusion strategies in patients with STEMI has significantly reduced the mortality rate over last decade. Despite this, the rate of 30-day mortality remains high and approximately 25% of surviving patients develop heart failure. One of the mechanisms responsible for these adverse outcomes is Reperfusion injury. Reperfusion injury refers to myocardial cell death secondary to restoration of blood flow to the ischemic myocardium. The absence of oxygen and nutrients from blood creates a condition in which the restoration of circulation results in inflammation and oxidative damage through the induction of oxidative stress rather than restoration of normal function.
Ischemia-reperfusion injury creates the base line for tissue damage and cellular apoptosis. The tissue damage follows a natural progression of cellular and metabolic events initiated by an ischemic episode. Ischemia induces various intracellular or extracellular changes leading ton increased calcium intracellularly and ATP depletion that will end up in the cell death if the ongoing process does not stopped. Reperfusion is considered as a stopper for this and leads to flushing of tissues with toxic metabolites , primarily reactive oxygen species . This leads to Increased mitochondrial pore permeability <math>\longrightarrow</math>complement activation & cytochrome release <math>\longrightarrow</math>Inflammation and edema <math>\longrightarrow</math>Neutrophil platelet adhesion and thrombosis leading to progressive tissue death.
Pathophysiology
Pathophysiological Mechanism is as follows:
- The pathophysiologic mechanisms underlying reperfusion injury includes various steps starting from infarction, inflammation, generation of free radicals, an increase in intracellular calcium, development of edema, mitochondrial damage and finally leading to activation of coagulation.
- Reperfusion damage occurs after myocardial reperfusion after a period of decreased oxygen supply. The damage from reperfusion injury is partially due to the affected tissue's inflammatory response. White blood cells transported to the region by fresh blood release a host of inflammatory factors such as interleukins and free radicals in response to tissue injury. Blood flow restored reintroduces oxygen inside cells that damages cellular proteins, DNA, and plasma membrane. Damage to the membrane of the cell will in effect cause further free radicals to be released. These reactive species can also act indirectly to turn on apoptosis through redox signaling. Also, leukocytes may build up in small capillaries, block them and cause more ischemia
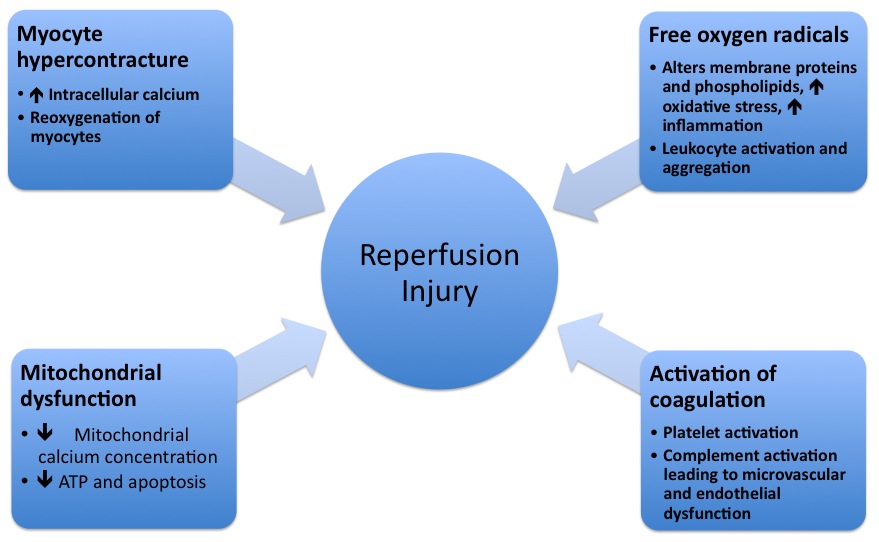
- Mitochondrial dysfunction plays a significant role in reperfusion injury. Although the mitochondrial membrane is normally impermeable to ions and metabolites, ischemia changes permeability by elevating concentrations of intro-mitochondrial calcium, reducing concentrations of adenine nucleotides, and inducing oxidative stress. It gives primacy to the mitochondrial transfer pore permeability (MPTP), which opens when reperfusion occurs. This contributes to increased osmotic load in the mitochondrial body causing swelling and breakup, releasing proteins that induce apoptosis from mitochondria. Mitochondrial activity is impaired, and ATP is hydrolyzed, allowing degrading enzymes to activate. Finally, excessive activation of the Poly ADP ribose polymerase-1 (PARP-1) impairs the work of other organelles and speeds up the development of reactive oxygen species.
- Hypoxanthine is produced as breakdown product of the ATP metabolism in prolonged ischemia (60 minutes or more). As a consequence of higher oxygen availability the enzyme xanthine dehydrogenase is converted to xanthine oxidase. This oxidation contributes to the conversion of molecular oxygen into highly reactive superoxide and hydroxyl radicals. Xanthine oxidase also produces uric acid, which can act both as a pro-oxidant and as a reactive species scavenger such as per-oxinitrite. To produce the potent reactive species per-oxinitrite, too much nitric oxide formed during reperfusion reacts with superoxide. These radicals and reactive oxygen species attack lipids , proteins, and glycosaminoglycan from the cell membrane, causing further damage. Specific biological processes may also be initiated by redox signaling.
Risk Factors
Risk factors for reperfusion injury include
- Hypertension with left ventricular hypertrophy,
- Congestive heart failure,
- Increased age,
- Diabetes, and
- Hyperlipidemia
Natural History, Complications and Prognosis
Reperfusion injury may be responsible for about 50% of the total infarct size after an acute myocardial infarction as well as myocardial stunning, congestive heart failure and reperfusion arrhythmias such as ventricular arrhythmias.[1]
Medical Therapy
While many pharmacotherapies are successful in limiting reperfusion injury in animal studies or ex-vivo, the majority have failed to improve clinical outcomes in randomized clinical trials in patients. Strategies may have failed as a result of targeting the wrong mechanism, because an inadequate dose was studied, because patients with insufficient potential for benefit were studied, and because the drug was administered too late (after reperfusion had already occurred).
References
|
Reperfusion injury Microchapters |
|
Treatment |
|---|
|
Reperfusion injury overview On the Web |
|
American Roentgen Ray Society Images of Reperfusion injury overview |
|
Risk calculators and risk factors for Reperfusion injury overview |