Ramelteon: Difference between revisions
Gloria Picoy (talk | contribs) No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 192: | Line 192: | ||
In a 35-night, double-blind, placebo-controlled, parallel-group study in adults with chronic insomnia, measures of residual effects were performed at three time points. Overall, the magnitudes of any observed differences were small. At Week 1, patients who received 8 mg of ROZEREM had a mean VAS score (46 mm on a 100 mm scale) indicating more fatigue in comparison to patients who received placebo (42 mm). At Week 3, patients who received 8 mg of ROZEREM had a lower mean score for immediate recall (7.5 out of 16 words) compared to patients who received placebo (8.2 words); and the patients treated with ROZEREM had a mean VAS score indicating more sluggishness (27 mm on a 100 mm VAS) in comparison to the placebo-treated patients (22 mm). Patients who received ROZEREM did not have next-morning residual effects that were different from placebo at Week 5. | In a 35-night, double-blind, placebo-controlled, parallel-group study in adults with chronic insomnia, measures of residual effects were performed at three time points. Overall, the magnitudes of any observed differences were small. At Week 1, patients who received 8 mg of ROZEREM had a mean VAS score (46 mm on a 100 mm scale) indicating more fatigue in comparison to patients who received placebo (42 mm). At Week 3, patients who received 8 mg of ROZEREM had a lower mean score for immediate recall (7.5 out of 16 words) compared to patients who received placebo (8.2 words); and the patients treated with ROZEREM had a mean VAS score indicating more sluggishness (27 mm on a 100 mm VAS) in comparison to the placebo-treated patients (22 mm). Patients who received ROZEREM did not have next-morning residual effects that were different from placebo at Week 5. | ||
======Rebound Insomnia/Withdrawal====== | |||
Potential rebound insomnia and withdrawal effects were assessed in four studies in which subjects received ROZEREM or placebo for up to 6 months; 3 were 35-day studies, one was a 6 month study. These studies included a total of 2533 subjects, of whom 854 were elderly. | Potential rebound insomnia and withdrawal effects were assessed in four studies in which subjects received ROZEREM or placebo for up to 6 months; 3 were 35-day studies, one was a 6 month study. These studies included a total of 2533 subjects, of whom 854 were elderly. | ||
Revision as of 15:40, 21 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ramelteon is a nonbarbiturate hypnotic that is FDA approved for the treatment of insomnia characterized by difficulty with sleep onset. Common adverse reactions include somnolence, dizziness, fatigue, nausea and exacerbated insomnia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Ramelteon is indicated for the treatment of insomnia characterized by difficulty with sleep onset.
- Dosage:
- Adult dose: 8 mg taken within 30 minutes of going to bed.
- Should not be taken with or immediately after a high-fat meal.
- Total daily dose should not exceed 8 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ramelteon in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ramelteon in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and efficacy not established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ramelteon in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ramelteon in pediatric patients.
Contraindications
- Patients who develop angioedema after treatment with ROZEREM should not be rechallenged with the drug.
- Patients should not take ROZEREM in conjunction with fluvoxamine
Warnings
Severe Anaphylactic and Anaphylactoid Reactions
- Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of ROZEREM.
- Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department.
- If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal.
- Patients who develop angioedema after treatment with ROZEREM should not be rechallenged with the drug.
Need to Evaluate for Co-morbid Diagnoses
- Since sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient.
- The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia, or the emergence of new cognitive or behavioral abnormalities, may be the result of an unrecognized underlying psychiatric or physical disorder and requires further evaluation of the patient.
- Exacerbation of insomnia and emergence of cognitive and behavioral abnormalities were seen with ROZEREM during the clinical development program.
Abnormal Thinking and Behavioral Changes
- A variety of cognitive and behavior changes have been reported to occur in association with the use of hypnotics.
- In primarily depressed patients, worsening of depression (including suicidal ideation and completed suicides) has been reported in association with the use of hypnotics.
- Hallucinations, as well as behavioral changes such as bizarre behavior, agitation and mania have been reported with ROZEREM use. Amnesia, anxiety and other neuro-psychiatric symptoms may also occur unpredictably.
- Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a hypnotic) and other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex), with amnesia for the event, have been reported in association with hypnotic use. The use of alcohol and other CNS depressants may increase the risk of such behaviors. These events can occur in hypnotic-naive as well as in hypnotic-experienced persons. Complex behaviors have been reported with the use of ROZEREM. Discontinuation of ROZEREM should be strongly considered for patients who report any complex sleep behavior.
CNS Effects
- Patients should avoid engaging in hazardous activities that require concentration (such as operating a motor vehicle or heavy machinery) after taking ROZEREM.
- After taking ROZEREM, patients should confine their activities to those necessary to prepare for bed.
- Patients should be advised not to consume alcohol in combination with ROZEREM as alcohol and ROZEREM may have additive effects when used in conjunction.
Reproductive Effects
Use in Adolescents and Children
- Ramelteon has been associated with an effect on reproductive hormones in adults, e.g., decreased testosterone levels and increased prolactin levels.
- It is not known what effect chronic or even chronic intermittent use of ROZEREM may have on the reproductive axis in developing humans.
Use in Patients with Concomitant Illness
- Ramelteon has not been studied in subjects with severe sleep apnea and is not recommended for use in this population.
- Ramelteon should not be used by patients with severe hepatic impairment.
Laboratory Tests
Monitoring
- No standard monitoring is required.
- For patients presenting with unexplained amenorrhea, galactorrhea, decreased libido, or problems with fertility, assessment of prolactin levels and testosterone levels should be considered as appropriate.
Interference with Laboratory Tests
- Ramelteon is not known to interfere with commonly used clinical laboratory tests.
- In addition, in vitro data indicate that ramelteon does not cause false-positive results for benzodiazepines, opiates, barbiturates, cocaine, cannabinoids, or amphetamines in two standard urine drug screening methods in vitro.
Adverse Reactions
Clinical Trials Experience
Adverse Reactions Resulting in Discontinuation of Treatment
- The data described in this section reflect exposure to ROZEREM in 5373 subjects, including 722 exposed for 6 months or longer, and 448 subjects for one year.
- Six percent of the 5373 individual subjects exposed to ROZEREM in clinical studies discontinued treatment owing to an adverse event, compared with 2% of the 2279 subjects receiving placebo. The most frequent adverse events leading to discontinuation in subjects receiving ROZEREM were somnolence, dizziness, nausea, fatigue, headache, and insomnia; all of which occurred in 1% of the patients or less.
Ramelteon Most Commonly Observed Adverse Events
- Table 1 displays the incidence of adverse events reported by the 2861 patients with chronic insomnia who participated in placebo-controlled trials of ROZEREM.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of other drugs, and may not reflect the rates observed in practice.
- The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.

Postmarketing Experience
There is limited information regarding Ramelteon Postmarketing Experience in the drug label.
Drug Interactions
Effects of Other Drugs on ROZEREM
- Fluvoxamine (strong CYP1A2 inhibitor): AUC0-inf for ramelteon increased approximately 190-fold, and the Cmax increased approximately 70-fold upon coadministration of fluvoxamine and ROZEREM, compared to ROZEREM administered alone. ROZEREM should not be used in combination with fluvoxamine. Other less strong CYP1A2 inhibitors have not been adequately studied. ROZEREM should be administered with caution to patients taking less strong CYP1A2 inhibitors.
- Rifampin (strong CYP enzyme inducer): Administration of multiple doses of rifampin resulted in a mean decrease of approximately 80% in total exposure to ramelteon and metabolite M-II. Efficacy may be reduced when ROZEREM is used in combination with strong CYP enzyme inducers such as rifampin.
- Ketoconazole (strong CYP3A4 inhibitor): The AUC0-inf and Cmax of ramelteon increased by approximately 84% and 36% upon coadministration of ketoconazole with ROZEREM. ROZEREM should be administered with caution in subjects taking strong CYP3A4 inhibitors such as ketoconazole.
- Fluconazole (strong CYP2C9 inhibitor): The AUC0-inf and Cmax of ramelteon was increased by approximately 150% when ROZEREM was coadministered with fluconazole. ROZEREM should be administered with caution in subjects taking strong CYP2C9 inhibitors such as fluconazole.
- Donepezil: The AUC0-inf and Cmax of ramelteon increased by approximately 100% and 87%, respectively upon coadministration of donepezil with ROZEREM. Patients should be closely monitored when ROZEREM is coadministered with donepezil.
- Doxepin: The AUC0-inf and Cmax of ramelteon increased by approximately 66% and 69%, respectively, upon coadministration of doxepin with ROZEREM. Patients should be closely monitored when ROZEREM is coadministered with doxepin.
Drug/Laboratory Test Interactions
- Ramelteon is not known to interfere with commonly used clinical laboratory tests. In addition, in vitro data indicate that ramelteon does not cause false-positive results for benzodiazepines, opiates, barbiturates, cocaine, cannabinoids, or amphetamines in two standard urine drug screening methods in vitro.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C In animal studies, ramelteon produced evidence of developmental toxicity, including teratogenic effects, in rats at doses much greater than the recommended human dose (RHD) of 8 mg/day. There are no adequate and well-controlled studies in pregnant women. ROZEREM should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Oral administration of ramelteon (10, 40, 150 or 600 mg/kg/day) to pregnant rats during the period of organogenesis was associated with increased incidences of fetal structural abnormalities (malformations and variations) at doses greater than 40 mg/kg/day. The no-effect dose is approximately 50 times the RHD on a body surface area (mg/m2) basis. Treatment of pregnant rabbits during the period of organogenesis produced no evidence of embryo-fetal toxicity at oral doses of up to 300 mg/kg/day (or up to 720 times the RHD on a mg/m2 basis.
When rats were orally administered ramelteon (30, 100, or 300 mg/kg/day) throughout gestation and lactation, growth retardation, developmental delay, and behavioral changes were observed in the offspring at doses greater than 30 mg/kg/day. The no-effect dose is 36 times the RHD on a mg/m2 basis. Increased incidences of malformation and death among offspring were seen at the highest dose.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ramelteon in women who are pregnant.
Labor and Delivery
The potential effects of ROZEREM on the duration of labor and/or delivery, for either the mother or the fetus, have not been studied. ROZEREM has no established use in labor and delivery.
Nursing Mothers
It is not known whether ramelteon is secreted into human milk; however ramelteon is secreted into the milk of lactating rats. Because many drugs are excreted into human milk, caution should be exercised when administered to a nursing woman.
Pediatric Use
Safety and effectiveness of ROZEREM in pediatric patients have not been established. Further study is needed prior to determining that this product may be used safely in pre-pubescent and pubescent patients.
Geriatic Use
A total of 654 subjects in double-blind, placebo-controlled, efficacy trials who received ROZEREM were at least 65 years of age; of these, 199 were 75 years of age or older. No overall differences in safety or efficacy were observed between elderly and younger adult subjects.
A double-blind, randomized, placebo-controlled study in elderly subjects with insomnia (n=33) evaluated the effect of a single dose of ROZEREM on balance, mobility, and memory functions after middle of the night awakening. There is no information on the effect of multiple dosing. Night time dosing of ROZEREM 8 mg did not impair middle of the night balance, mobility, or memory functions relative to placebo. The effects on night balance in the elderly cannot be definitively known from this study.
Gender
There is no FDA guidance on the use of Ramelteon with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ramelteon with respect to specific racial populations.
Renal Impairment
No effects on Cmax and AUC0-t of parent drug or M-II were seen. No adjustment of ROZEREM dosage is required in patients with renal impairment.
Hepatic Impairment
Exposure to ROZEREM was increased by 4-fold in subjects with mild hepatic impairment and by more than 10-fold in subjects with moderate hepatic impairment. ROZEREM should be used with caution in patients with moderate hepatic impairment. ROZEREM is not recommended in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ramelteon in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ramelteon in patients who are immunocompromised.
Sleep Apnea
The effects of ROZEREM were evaluated after administering a 16 mg dose or placebo in a crossover design to subjects (n=26) with mild to moderate obstructive sleep apnea. Treatment with ROZEREM 16 mg for one night showed no difference compared with placebo on the Apnea/Hypopnea Index (the primary outcome variable), apnea index, hypopnea index, central apnea index, mixed apnea index, and obstructive apnea index. Treatment with a single dose of ROZEREM does not exacerbate mild to moderate obstructive sleep apnea. There is no available information on the respiratory effects of multiple doses of ROZEREM in patients with sleep apnea. The effects on exacerbation in patients with mild to moderate sleep apnea cannot be definitively known from this study.
ROZEREM has not been studied in subjects with severe obstructive sleep apnea; use of ROZEREM is not recommended in such patients.
Chronic Obstructive Pulmonary Disease
The respiratory depressant effect of ROZEREM was evaluated in a crossover design study of subjects (n=26) with mild to moderate COPD after administering a single 16 mg dose or placebo, and in a separate study (n=25), the effects of ROZEREM on respiratory parameters were evaluated after administering an 8 mg dose or placebo in a crossover design to patients with moderate to severe COPD, defined as patients who had forced expiratory volume at one second (FEV1)/forced vital capacity ratio of <70%, and a FEV1 <80% of predicted with <12% reversibility to albuterol. Treatment with a single dose of ROZEREM has no demonstrable respiratory depressant effects in subjects with mild to severe COPD, as measured by arterial O2 saturation (SaO2). There is no available information on the respiratory effects of multiple doses of ROZEREM in patients with COPD. The respiratory depressant effects in patients with COPD cannot be definitively known from this study.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Ramelteon Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ramelteon and IV administrations.
Overdosage
- General symptomatic and supportive measures should be used, along with immediate gastric lavage where appropriate.
- Intravenous fluids should be administered as needed.
- As in all cases of drug overdose, respiration, pulse, blood pressure, and other appropriate vital signs should be monitored, and general supportive measures employed.
- Hemodialysis does not effectively reduce exposure to ROZEREM. Therefore, the use of dialysis in the treatment of overdosage is not appropriate.
Pharmacology
There is limited information regarding Ramelteon Pharmacology in the drug label.
Mechanism of Action
Ramelteon is a melatonin receptor agonist with both high affinity for melatonin MT1 and MT2 receptors and selectivity over the MT3 receptor. Ramelteon demonstrates full agonist activity in vitro in cells expressing human MT1 or MT2 receptors.
The activity of ramelteon at the MT1 and MT2 receptors is believed to contribute to its sleep-promoting properties, as these receptors, acted upon by endogenous melatonin, are thought to be involved in the maintenance of the circadian rhythm underlying the normal sleep-wake cycle.
Ramelteon has no appreciable affinity for the GABA receptor complex or for receptors that bind neuropeptides, cytokines, serotonin, dopamine, noradrenaline, acetylcholine, and opiates. Ramelteon also does not interfere with the activity of a number of selected enzymes in a standard panel.
The major metabolite of ramelteon, M-II, is active and has approximately one tenth and one fifth the binding affinity of the parent molecule for the human MT1 and MT2 receptors, respectively, and is 17- to 25-fold less potent than ramelteon in in vitro functional assays. Although the potency of M-II at MT1 and MT2 receptors is lower than the parent drug, M-II circulates at higher concentrations than the parent producing 20- to 100-fold greater mean systemic exposure when compared to ramelteon. M-II has weak affinity for the serotonin 5-HT2B receptor, but no appreciable affinity for other receptors or enzymes. Similar to ramelteon, M-II does not interfere with the activity of a number of endogenous enzymes.
All other known metabolites of ramelteon are inactive.
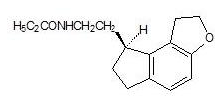
Structure
The compound is produced as the (S)-enantiomer, with an empirical formula of C16H21NO2, molecular weight of 259.34, and the following chemical structure:
Pharmacodynamics
There is limited information regarding Ramelteon Pharmacodynamics in the drug label.
Pharmacokinetics
The pharmacokinetic profile of ROZEREM has been evaluated in healthy subjects as well as in subjects with hepatic or renal impairment. When administered orally to humans in doses ranging from 4 to 64 mg, ramelteon undergoes rapid, high first-pass metabolism, and exhibits linear pharmacokinetics. Maximal serum concentration (Cmax) and area under the concentration-time curve (AUC) data show substantial intersubject variability, consistent with the high first-pass effect; the coefficient of variation for these values is approximately 100%. Several metabolites have been identified in human serum and urine.
Absorption
Ramelteon is absorbed rapidly, with median peak concentrations occurring at approximately 0.75 hour (range, 0.5 to 1.5 hours) after fasted oral administration. Although the total absorption of ramelteon is at least 84%, the absolute oral bioavailability is only 1.8% due to extensive first-pass metabolism.
Distribution
In vitro protein binding of ramelteon is approximately 82% in human serum, independent of concentration. Binding to albumin accounts for most of that binding, since 70% of the drug is bound in human serum albumin. Ramelteon is not distributed selectively to red blood cells.
Ramelteon has a mean volume of distribution after intravenous administration of 73.6 L, suggesting substantial tissue distribution.
Metabolism
Metabolism of ramelteon consists primarily of oxidation to hydroxyl and carbonyl derivatives, with secondary metabolism producing glucuronide conjugates. CYP1A2 is the major isozyme involved in the hepatic metabolism of ramelteon; the CYP2C subfamily and CYP3A4 isozymes are also involved to a minor degree.
The rank order of the principal metabolites by prevalence in human serum is M-II, M-IV, M-I, and M-III. These metabolites are formed rapidly and exhibit a monophasic decline and rapid elimination. The overall mean systemic exposure of M-II is approximately 20- to 100-fold higher than parent drug.
Elimination
Following oral administration of radiolabeled ramelteon, 84% of total radioactivity was excreted in urine and approximately 4% in feces, resulting in a mean recovery of 88%. Less than 0.1% of the dose was excreted in urine and feces as the parent compound. Elimination was essentially complete by 96 hours post-dose.
Repeated once daily dosing with ROZEREM does not result in significant accumulation owing to the short elimination half-life of ramelteon (on average, approximately 1 to 2.6 hours).
The half-life of M-II is 2 to 5 hours and independent of dose. Serum concentrations of the parent drug and its metabolites in humans are at or below the lower limits of quantitation within 24 hours.
Effect of Food
When administered with a high-fat meal, the AUC0-inf for a single 16 mg dose of ROZEREM was 31% higher and the Cmax was 22% lower than when given in a fasted state. Median Tmax was delayed by approximately 45 minutes when ROZEREM was administered with food. Effects of food on the AUC values for M-II were similar. It is therefore recommended that ROZEREM not be taken with or immediately after a high-fat meal.
Nonclinical Toxicology
Carcinogenesis
Ramelteon was administered to mice and rats at oral doses of 0, 30, 100, 300, or 1000 mg/kg/day (mice) and 0, 15, 60, 250, or 1000 mg/kg/day (rats). Mice and rats were dosed for two years, except at the high dose (94 weeks for male and female mice and female rats). In mice, dose-related increases in the incidence of hepatic tumors (adenomas, carcinomas, hepatoblastomas) were observed in males and females. The no-effect dose for hepatic tumors in mice (30 mg/kg/day) is approximately 20 times the recommended human dose (RHD) of 8 mg/day on a body surface area (mg/m2) basis.
In rats, the incidence of hepatic adenoma and benign Leydig cell tumors of the testis was increased in males at doses ≥250 mg/kg/day. In females, the incidence of hepatic adenoma was increased at doses ≥60 mg/kg/day. The incidence of hepatic carcinoma was increased in males and female rats at 1000 mg/kg/day. The no-effect dose for tumors in rats (15 mg/kg/day) is approximately 20 times the RHD on a mg/m2 basis.
Mutagenesis
Ramelteon was not genotoxic in the in vitro bacterial reverse mutation (Ames) assay, the in vitro mouse lymphoma TK+/- assay, and in in vivo oral micronucleus assays in mouse and rat. Ramelteon was clastogenic in the in vitro chromosomal aberration assay in Chinese hamster lung cells.
Separate studies indicated that the concentration of the M-II metabolite formed in the presence of metabolic activation exceeded the concentration of ramelteon; therefore, the genotoxic potential of the M-II metabolite was also assessed in the in vitro studies.
Clinical Studies
Controlled Clinical Trials
Chronic Insomnia
Three randomized, double-blind trials in subjects with chronic insomnia employing polysomnography (PSG) were provided as objective support of Rozerem's effectiveness in sleep initiation.
One study enrolled younger adults (aged 18 to 64 years, inclusive) with chronic insomnia and employed a parallel design in which the subjects received a single, nightly dose of ROZEREM (8 mg or 16 mg) or matching placebo for 35 days. PSG was performed on the first two nights in each of Weeks 1, 3, and 5 of treatment. ROZEREM reduced the average latency to persistent sleep at each of the time points when compared to placebo. The 16 mg dose conferred no additional benefit for sleep initiation.
The second study employing PSG was a three-period crossover trial performed in subjects aged 65 years and older with a history of chronic insomnia. Subjects received ROZEREM (4 mg or 8 mg) or placebo and underwent PSG assessment in a sleep laboratory for two consecutive nights in each of the three study periods. Both doses of ROZEREM reduced latency to persistent sleep when compared to placebo.
The third study evaluated long term efficacy and safety in adults with chronic insomnia. Subjects received a single, nightly dose of ROZEREM 8 mg or matching placebo for 6 months. PSG was performed on the first two nights of Week 1 and Months 1, 3, 5, and 6. ROZEREM reduced sleep latency at each time point when compared to placebo. In this study, when the PSG results from nights 1 and 2 of Month 7 were compared to the results from nights 22 and 23 of Month 6, there was a statistically significant increase in LPS of 33% (9.5 minutes) in the ramelteon group. There was no increase in LPS in the placebo group when the same time periods were compared.
A randomized, double-blind, parallel group study was conducted in outpatients aged 65 years and older with chronic insomnia and employed subjective measures of efficacy (sleep diaries). Subjects received ROZEREM (4 mg or 8 mg) or placebo for 35 nights. ROZEREM reduced patient-reported sleep latency compared to placebo. A similarly designed study performed in younger adults (aged 18 - 64 years) using 8 mg and 16 mg of ramelteon did not replicate this finding of reduced patient-reported sleep latency compared to placebo.
While the 16 mg dose was evaluated as a potential treatment for adults, it was shown to confer no additional benefit for sleep initiation and was associated with higher incidences of fatigue, headache and next-day somnolence.
Transient Insomnia
In a randomized, double-blind, parallel-group trial using a first-night-effect model, healthy adults received placebo or ROZEREM before spending one night in a sleep laboratory and being evaluated with PSG. ROZEREM demonstrated a decrease in mean latency to persistent sleep as compared to placebo.
Studies Pertinent to Safety Concerns for Sleep- promoting Drugs
Results from Human Laboratory Abuse Liability Studies
A human laboratory abuse potential study was performed in 14 subjects with a history of sedative/hypnotic or anxiolytic drug abuse. Subjects received single oral doses of ROZEREM (16, 80, or 160 mg), triazolam (0.25, 0.50, or 0.75 mg) or placebo. All subjects received each of the 7 treatments separated by a wash-out period and underwent multiple standard tests of abuse potential. No differences in subjective responses indicative of abuse potential were found between ROZEREM and placebo at doses up to 20 times the recommended therapeutic dose. The positive control drug, triazolam, consistently showed a dose-response effect on these subjective measures, as demonstrated by the differences from placebo in peak effect and overall 24-hour effect.
Residual Pharmacological Effect in Insomnia Trials
In order to evaluate potential next-day residual effects, the following scales were used: a Memory Recall Test, a Word List Memory Test, a Visual Analog Mood and Feeling Scale, the Digit-Symbol Substitution Test, and a post-sleep questionnaire to assess alertness and ability to concentrate. There was no evidence of next-day residual effect seen after 2 nights of ramelteon use during the crossover studies.
In a 35-night, double-blind, placebo-controlled, parallel-group study in adults with chronic insomnia, measures of residual effects were performed at three time points. Overall, the magnitudes of any observed differences were small. At Week 1, patients who received 8 mg of ROZEREM had a mean VAS score (46 mm on a 100 mm scale) indicating more fatigue in comparison to patients who received placebo (42 mm). At Week 3, patients who received 8 mg of ROZEREM had a lower mean score for immediate recall (7.5 out of 16 words) compared to patients who received placebo (8.2 words); and the patients treated with ROZEREM had a mean VAS score indicating more sluggishness (27 mm on a 100 mm VAS) in comparison to the placebo-treated patients (22 mm). Patients who received ROZEREM did not have next-morning residual effects that were different from placebo at Week 5.
Rebound Insomnia/Withdrawal
Potential rebound insomnia and withdrawal effects were assessed in four studies in which subjects received ROZEREM or placebo for up to 6 months; 3 were 35-day studies, one was a 6 month study. These studies included a total of 2533 subjects, of whom 854 were elderly.
Tyrer Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ): The BWSQ is a self-report questionnaire that solicits specific information on 20 symptoms commonly experienced during withdrawal from benzodiazepine receptor agonists; ROZEREM is not a benzodiazepine receptor agonist.
In two of the three 35-day insomnia studies, the questionnaire was administered one week after completion of treatment; in the third study, the questionnaire was administered on Days 1 and 2 after completion. In all three of the 35-day studies, subjects receiving ROZEREM 4 mg, 8 mg, or 16 mg daily reported BWSQ scores similar to those of subjects receiving placebo.
In the 6 month study, there was no evidence of withdrawal from the 8 mg dose as measured by the BWSQ.
Rebound Insomnia: Rebound insomnia was assessed in the 35-day studies by measuring sleep latency after abrupt treatment discontinuation. One of these studies employed PSG in younger adult subjects receiving ROZEREM 8 mg or 16 mg; the other two studies employed subjective measures of sleep-onset insomnia in elderly subjects receiving ROZEREM 4 mg or 8 mg, and in younger adult subjects receiving ROZEREM 8 mg or 16 mg. There was no evidence that ROZEREM caused rebound insomnia during the post-treatment period.
Studies to Evaluate Effects on Endocrine Function
Two controlled studies evaluated the effects of ROZEREM on endocrine function.
In the first trial, ROZEREM 16 mg once daily or placebo was administered to 99 healthy volunteer subjects for 4 weeks. This study evaluated the thyroid axis, adrenal axis and reproductive axis. No clinically significant endocrinopathies were demonstrated in this study. However, the study was limited in its ability to detect such abnormalities due to its limited duration.
In the second trial, ROZEREM 16 mg once daily or placebo was administered to 122 subjects with chronic insomnia for 6 months. This study evaluated the thyroid axis, adrenal axis and reproductive axis. There were no significant abnormalities seen in either the thyroid or the adrenal axes. Abnormalities were, however, noted within the reproductive axis. Overall, the mean serum prolactin level change from baseline was 4.9 mcg/L (34% increase) for women in the ROZEREM group compared with -0.6 mcg/L (4% decrease) for women in the placebo group (p=0.003). No differences between active- and placebo-treated groups occurred among men. Thirty-two percent of all patients who were treated with ramelteon in this study (women and men) had prolactin levels that increased from normal baseline levels compared to 19% of patients who were treated with placebo. Subject-reported menstrual patterns were similar between the two treatment groups.
In a 12-month, open-label study in adult and elderly patients, there were two patients who were noted to have abnormal morning cortisol levels, and subsequent abnormal ACTH stimulation tests. A 29-year-old female patient was diagnosed with a prolactinoma. The relationship of these events to ROZEREM therapy is not clear.
How Supplied
There is limited information regarding Ramelteon How Supplied in the drug label.
Storage
There is limited information regarding Ramelteon Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ramelteon |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ramelteon |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ramelteon Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol by itself impairs performance and can cause sleepiness. Since the intended effect of ROZEREM is to promote sleep, patients should be cautioned not to consume alcohol when using ROZEREM. Use of the products in combination may have an additive effect.
Brand Names
There is limited information regarding Ramelteon Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ramelteon Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Ramelteon.svg | |
| File:Ramelteon3d.png | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 1.8% |
| Protein binding | ~82% |
| Metabolism | Hepatic (CYP1A2-mediated) |
| Elimination half-life | 1-2.6 hours |
| Excretion | Renal (84%) and fecal (4%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H21NO2 |
| Molar mass | 259.343 g/mol |
For patient information please see the page entitled Ramelteon (patient information)
Ramelteon, marketed as Rozerem by Takeda Pharmaceuticals North America, is the first in a new class of sleep agents that selectively binds to the melatonin receptors in the suprachiasmatic nucleus (SCN), versus binding to GABA A receptors, such as with drugs like zolpidem, eszopiclone, and zaleplon. Ramelteon is approved by the FDA for long-term use.
Ramelteon does not show any appreciable binding to GABAA receptors, which are associated with anxiolytic, myorelaxant, and amnesic effects.
Uses
Ramelteon is used for insomnia, particularly delayed sleep onset. Ramelteon has not been shown to produce dependence and has shown no potential for abuse, and the withdrawal and rebound insomnia that is typical with other GABA modulators is not present in ramelteon. It is currently the only non-scheduled prescription drug for the treatment of insomnia available in the United States.[1]
Mechanism of action
Ramelteon is a melatonin receptor agonist with both high affinity for melatonin MT1 and MT2 receptors and selectivity over the MT3 receptor. Ramelteon demonstrates full agonist activity in vitro in cells expressing human MT1 or MT2 receptors, and high selectivity for human MT 1 and MT2 receptors compared to the MT3 receptor.
The activity of ramelteon at the MT1 and MT2 receptors is believed to contribute to its sleep-promoting properties, as these receptors, acted upon by endogenous melatonin, are thought to be involved in the maintenance of the circadian rhythm underlying the normal sleep-wake cycle. Ramelteon has no appreciable affinity for the GABA receptor complex or for receptors that bind neuropeptides, cytokines, serotonin, dopamine, noradrenaline, acetylcholine, and opiates. Ramelteon also does not interfere with the activity of a number of selected enzymes in a standard panel.
The significance of ramelteon's lack of affinity for the MT3 receptor is not clear, despite the manufacturer's emphasis of this fact in commercial advertisements. The MT3 receptor appears almost exclusively in the gut and might not have any relationship to sleep or wakefulness.
The major metabolite of ramelteon, M-II, is active and has approximately one tenth and one fifth the binding affinity of the parent molecule for the human MT1 and MT2 receptors, respectively, and is 17 – 25-fold less potent than ramelteon in in vitro functional assays. Although the potency of M-II at MT1 and MT2 receptors is lower than the parent drug, M-II circulates at higher concentrations than the parent producing 20 – 100 fold greater mean systemic exposure when compared to ramelteon. M-II has weak affinity for the serotonin 5-HT2B receptor, but no appreciable affinity for other receptors or enzymes. Similar to ramelteon, M-II does not interfere with the activity of a number of endogenous enzymes.
All other known metabolites of ramelteon are inactive.
No published studies have indicated whether ramelteon is more or less safe or effective than melatonin, a much less expensive drug, widely available in the United States without a prescription. The biological action of melatonin is similar to that of ramelteon. The purported advantage of ramelteon is that the product and dosage is more likely to be pure and standardized, because it is a prescription drug that is monitored by the Food and Drug Administration (FDA).
Drug interactions
Ramelteon has been studied in combination with omeprazole, theophylline, dextromethorphan, midazolam (although one would probably not come across this medicine due to its high clearance and shorter half-life than other benzodiazepines. Thus, Intensive Care Units must assess the patient and carefully administer as needed. It is also used as a sedative before operations. Its half-life is less or greater than one hour. It is usually used in an Intensive Care Unit and administered via IV due to the destructive nature of first pass metabolism.) Other such drug-drug interactions include digoxin and warfarin. There were no clinically meaningful effects when ramelteon was coadministered with any of these drugs.
A drug interaction study showed that there were no clinically meaningful effects or an increase in adverse events when ramelteon and the SSRI Prozac (fluoxetine) were coadministered. Ramelteon and fluvoxamine should not be coadministered.
Ramelteon should be administered with caution in patients taking other CYP1A2 inhibitors, strong CYP3A4 inhibitors such as ketoconazole, and strong CYP2C9 inhibitors such as fluconazole.
Efficacy may be reduced when ramelteon is used in combination with potent CYP enzyme inducers such as rifampin, since ramelteon concentrations may be decreased.
References
Sources and external links
- Pages with script errors
- Pages with broken file links
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Sedatives
- Drugs