Potassium bicarbonate: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Potassium bicarbonate": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (6 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{RB}} | |||
|genericName=Potassium bicarbonate | |||
|aOrAn=a | |aOrAn=a | ||
|drugClass=[[Potassium|potassium supplement]] | |||
|indicationType=treatment | |indicationType=treatment | ||
| | |indication=[[hypokalemia]], [[Digoxin|digitalis intoxication]], [[Hypokalemic periodic paralysis|hypokalemic familial periodic paralysis]] | ||
|adverseReactions=<!--Black Box Warning--> | |adverseReactions=[[nausea]], [[vomiting]], [[flatulence]], [[abdominal pain]]/[[discomfort]] and [[diarrhea]] | ||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult===== | |fdaLIADAdult=====Indications==== | ||
* For the treatment of patients with [[hypokalemia]], with or without [[metabolic alkalosis]]; in [[Digoxin|digitalis intoxication]]; and in patients with [[Hypokalemic periodic paralysis|hypokalemic familial periodic paralysis]]. If [[hypokalemia]] is the result of diuretic therapy, consideration should be given to the use of a lower dose of [[diuretic]], which may be sufficient without leading to [[hypokalemia]]. | |||
* | * For the prevention of [[hypokalemia]] in patients who would be at particular risk if [[hypokalemia]] were to develop (e.g., digitalized patients or patients with significant [[cardiac arrhythmias]]). | ||
* The use of [[potassium]] salts in patients receiving diuretics for uncomplicated essential [[hypertension]] is often unnecessary when such patients have a normal dietary pattern and when low doses of the diuretic are used. Serum potassium should be checked periodically, however, and if [[hypokalemia]] occurs, dietary supplementation with potassium-containing foods may be adequate to control milder cases. In more severe cases, and if dose adjustment of the diuretic is ineffective or unwarranted, supplementation with potassium salts may be indicated. | |||
* | |||
====Dosage==== | |||
* The usual dietary [[potassium]] intake by the average adult is 50 to 100 mEq per day. [[Potassium]] depletion sufficient to cause [[hypokalemia]] usually requires the loss of 200 mEq or more of [[potassium]] from the total body store. | |||
* | * Dosage must be adjusted to the individual needs of each patient. The dose for the prevention of [[hypokalemia]] is typically 25 mEq per day. Doses of 50 to 100 mEq per day or more are used for the treatment of [[potassium]] depletion. Dosage should be divided if more than 25 mEq per day is given such that no more than 25 mEq is given in a single dose. | ||
* | * The usual adult dose is 25 to 100 mEq of potassium per day (one KLOR-CON®/EF tablet 1 to 4 times daily after meals). | ||
* | * Each KLOR-CON®/EF tablet should be dissolved in at least 4 ounces of cold or ice water. These preparations, like other [[potassium]] supplements, must be properly diluted to avoid the possibility of gastrointestinal irritation. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=* Safety and effectiveness in children have not been established. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
* | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=* Potassium supplements are contraindicated in patients with [[hyperkalemia]] since a further increase in serum potassium concentration in such patients can produce [[cardiac arrest]]. [[Hyperkalemia]] may complicate any of the following conditions: chronic renal failure, systemic acidosis such as [[diabetic acidosis]], acute [[dehydration]], extensive tissue breakdown as in severe [[burns]], [[adrenal insufficiency]] or the administration of a potassium-sparing diuretic (e.g., [[spironolactone]], [[triamterene]] or [[amiloride]]) | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings======Hyperkalemia===== | ||
==== | |||
* In patients with impaired mechanisms for excreting potassium, the administration of potassium salts can produce [[hyperkalemia]] and [[cardiac arrest]]. This occurs most commonly in patients given potassium by the intravenous route but may also occur in patients given potassium orally. Potentially fatal [[hyperkalemia]] can develop rapidly and be asymptomatic. The use of potassium salts in patients with chronic renal disease, or any other condition which impairs potassium excretion, requires particularly careful monitoring of the serum potassium concentration and appropriate dosage adjustment. | |||
===== | =====Interaction with Potassium-Sparing Diuretics===== | ||
* [[Hypokalemia]] should not be treated by the concomitant administration of potassium salts and a potassium-sparing diuretic (e.g., [[spironolactone]], [[triamterene]] or [[amiloride]]), since the simultaneous administration of these agents can produce severe [[hyperkalemia]]. | |||
=====Interaction with Angiotensin Converting Enzyme Inhibitors===== | |||
* [[Angiotensin converting enzyme]] (ACE) inhibitors (e.g., [[captopril]], [[enalapril]]) will produce some potassium retention by inhibiting [[aldosterone]] production. [[Potassium]] supplements should be given to patients receiving [[ACE inhibitors]] only with close monitoring. | |||
=====Metabolic Acidosis===== | |||
* [[Hypokalemia]] in patients with [[metabolic acidosis]] should be treated with an alkalinizing potassium salt such as potassium bicarbonate, potassium citrate, [[potassium acetate]] or potassium gluconate. | |||
====Precautions==== | |||
===== | =====General===== | ||
* The diagnosis of [[potassium]] depletion is ordinarily made by demonstrating [[hypokalemia]] in a patient with a clinical history suggesting some cause for [[potassium]] depletion. In interpreting the serum [[potassium]] level, the physician should be aware that acute [[alkalosis]] per se can produce hypokalemia in the absence of a deficit in total body potassium while acute acidosis per se can increase the serum potassium concentration into the normal range even in the presence of a reduced total body potassium. The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease or acidosis, requires careful attention to acid-base balance and appropriate monitoring of serum electrolytes, the electrocardiogram and the clinical status of the patient. | |||
=====Information for Patients===== | |||
* Remind the patient to take this medicine following the frequency and amount prescribed. This is especially important if the patient is also taking diuretics and/or digitalis preparations. | |||
=====Laboratory Tests===== | |||
* When blood is drawn for analysis of plasma potassium, artifactual elevations can occur after improper [[venipuncture]] technique or as a result of in vitro hemolysis of the sample. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials=* One of the most severe adverse effects is [[hyperkalemia]] | |||
= | |||
* The most common adverse reactions to oral potassium salts are [[nausea]], [[vomiting]], [[flatulence]], [[abdominal pain]]/discomfort and [[diarrhea]]. These symptoms are due to irritation of the gastrointestinal tract and are best managed by diluting the preparation further, taking the dose with meals or reducing the dose. | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=* | |drugInteractions=* Potassium-sparing diuretics, angiotensin converting enzyme inhibitors | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA=* | |FDAPregCat=C | ||
|useInPregnancyFDA=* Animal reproduction studies have not been conducted with potassium salts. It is not known if potassium salts cause fetal harm when administered to a pregnant woman or affect reproductive capacity. Potassium supplements should be given to a pregnant woman only if clearly needed. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* Many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from oral potassium supplements, a decision should be made whether to discontinue nursing or discontinue the drug, considering the importance of the drug to the mother. | ||
|useInPed= | |useInPed=Safety and effectiveness in children have not been established. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
| Line 264: | Line 103: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* [[Oral]] | ||
|monitoring=* The use of potassium salts in patients with chronic renal disease, or any other condition which impairs potassium excretion, requires particularly careful monitoring of the serum potassium concentration and appropriate dosage adjustment. | |||
* Potassium supplements should be given to patients receiving [[ACE inhibitors]] only with close monitoring. | |||
|monitoring= | * The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease or acidosis, requires careful attention to acid-base balance and appropriate monitoring of serum electrolytes, the electrocardiogram and the clinical status of the patient. | ||
* | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=* The administration of oral potassium salts to persons with normal excretory mechanisms for [[potassium]] rarely causes serious [[hyperkalemia]]. However, if excretory mechanisms are impaired or if potassium is administered too rapidly intravenously, potentially fatal [[hyperkalemia]] can result. It is important to recognize that [[hyperkalemia]] is usually asymptomatic and may be manifested only by an increased serum potassium concentration (6.5-8.0 mEq/L) and characteristic electrocardiographic changes (peaking of T-waves, loss of P-wave, depression of S-T segment and prolongation of the QT interval). Late manifestations include [[muscle paralysis]] and [[cardiovascular collapse]] from [[cardiac arrest]] (9-12 mEq/L). | ||
* Treatment measures for [[hyperkalemia]] include the following: 1) elimination of foods and medications containing potassium and elimination of potassium-sparing diuretics; 2) [[intravenous]] administration of 300 to 500 mL/hr of 10% dextrose solution containing 10 to 20 units of crystalline insulin per 1,000 mL; 3) correction of acidosis, if present, with intravenous sodium bicarbonate; 4) use of exchange resins, [[hemodialysis]] or [[peritoneal dialysis]]. | |||
==== | * In treating hyperkalemia in patients who have been stabilized on digitalis, too rapid a lowering of the serum potassium concentration can produce digitalis toxicity. | ||
|drugBox={{chembox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 472266252 | |||
| Name = Potassium bicarbonate | |||
| ImageFile = Pot B Wiki str.png | |||
| ImageSize = 200px | |||
| ImageName = Potassium bicarbonate | |||
| IUPACName = potassium hydrogen carbonate | |||
| OtherNames = potassium acid carbonate | |||
| Section1 = {{Chembox Identifiers | |||
| CASNo = 298-14-6 | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| EINECS = 206-059-0 | |||
| PubChem = 516893 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 55053 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 81862 | |||
| SMILES = [K+].[O-]C(=O)O | |||
| InChI = 1/CH2O3.K/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-1 | |||
| InChIKey = TYJJADVDDVDEDZ-REWHXWOFAA | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/CH2O3.K/c2-1(3)4;/h(H2,2,3,4);/q;+1/p-1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = TYJJADVDDVDEDZ-UHFFFAOYSA-M | |||
| ATCCode_prefix = A12 | |||
| ATCCode_suffix = BA04 | |||
}} | |||
| Section2 = {{Chembox Properties | |||
| Formula = KHCO<sub>3</sub> | |||
| Appearance = white crystals | |||
| Odor = odorless | |||
| MolarMass = 100.115 g/mol | |||
| Density = 2.17 g/cm<sup>3</sup> | |||
| Solubility = 33.7 g/100 mL (20 °C) <br/> 60 g/100 mL (60 °C) | |||
| SolubleOther = practically insoluble in [[alcohol]] | |||
| MeltingPtC = 292 | |||
| Melting_notes = (decomposes) | |||
| pKa = 10.329<ref name="crc84">{{cite book|last1=Goldberg|first1=Robert N.|last2=Kishore|first2=Nand|last3=Lennen|first3=Rebecca M.|editor=David R. Lide|title=CRC handbook of chemistry and physics|url=http://books.google.com/books?id=q2qJId5TKOkC&pg=PP9|accessdate=6 March 2011|edition=84th|year=2003|publisher=CRC Press|location=Boca Raton, FL|isbn=978-0-8493-0595-5|pages=7–13|contribution=Thermodynamic quantities for the ionization reactions of buffers in water}}</ref> | |||
6.351 (carbonic acid)<ref name=crc84/> | |||
}} | |||
| Section4 = {{Chembox Thermochemistry | |||
| DeltaHf = -963.2 kJ/mol | |||
}} | |||
| Section7 = {{Chembox Hazards | |||
| ExternalMSDS = [http://msds.ehs.cornell.edu/msds/msdsdod/a495/m247034.htm MSDS] | |||
| EUIndex = Not listed | |||
| MainHazards = | |||
| NFPA-H = 1 | |||
| NFPA-F = 0 | |||
| NFPA-R = 0 | |||
| FlashPt = Non-Flammable | |||
| RPhrases = {{R36}} {{R37}} {{R38}} | |||
| SPhrases = | |||
| LD50 = > 2000 mg/kg (rat, oral) | |||
}} | |||
| Section8 = {{Chembox Related | |||
| OtherAnions = [[Potassium carbonate]] | |||
| OtherCations = [[Sodium bicarbonate]]<br/>[[Ammonium bicarbonate]] | |||
| OtherCpds = [[Potassium bisulfate]]<br/>[[Potassium hydrogen phosphate]] | |||
}} | |||
}} | |||
* | <!--Mechanism of Action--> | ||
|mechAction=* | |||
= | <!--Structure--> | ||
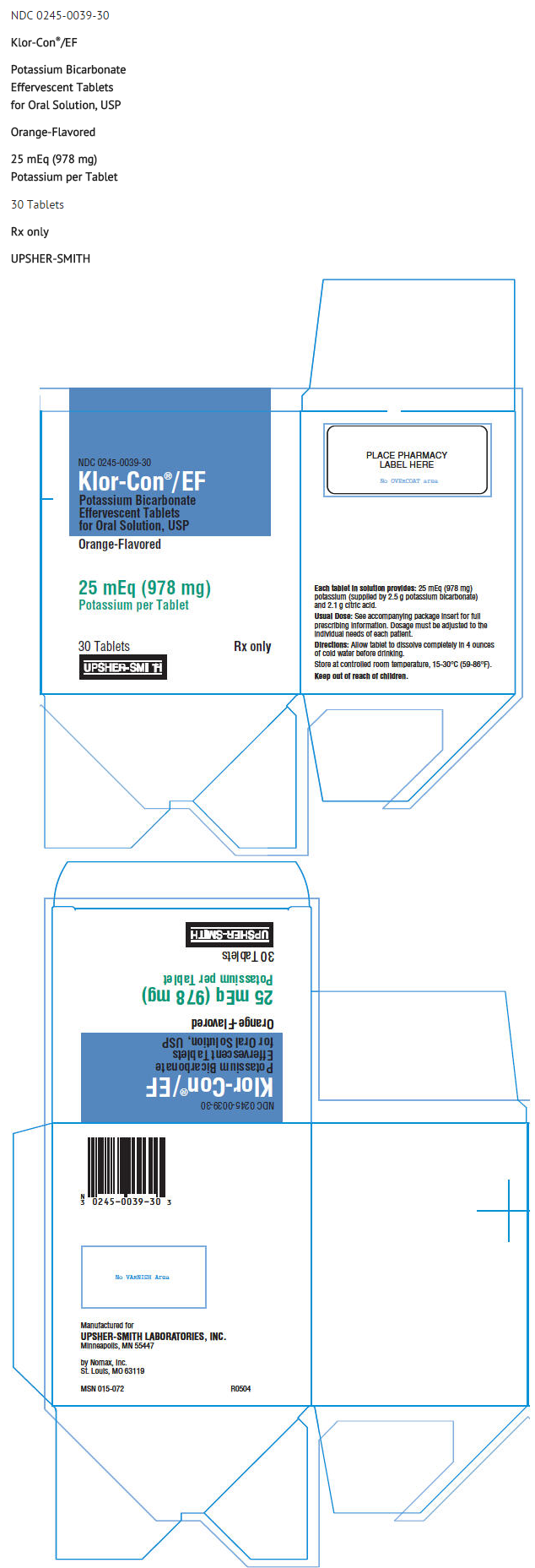
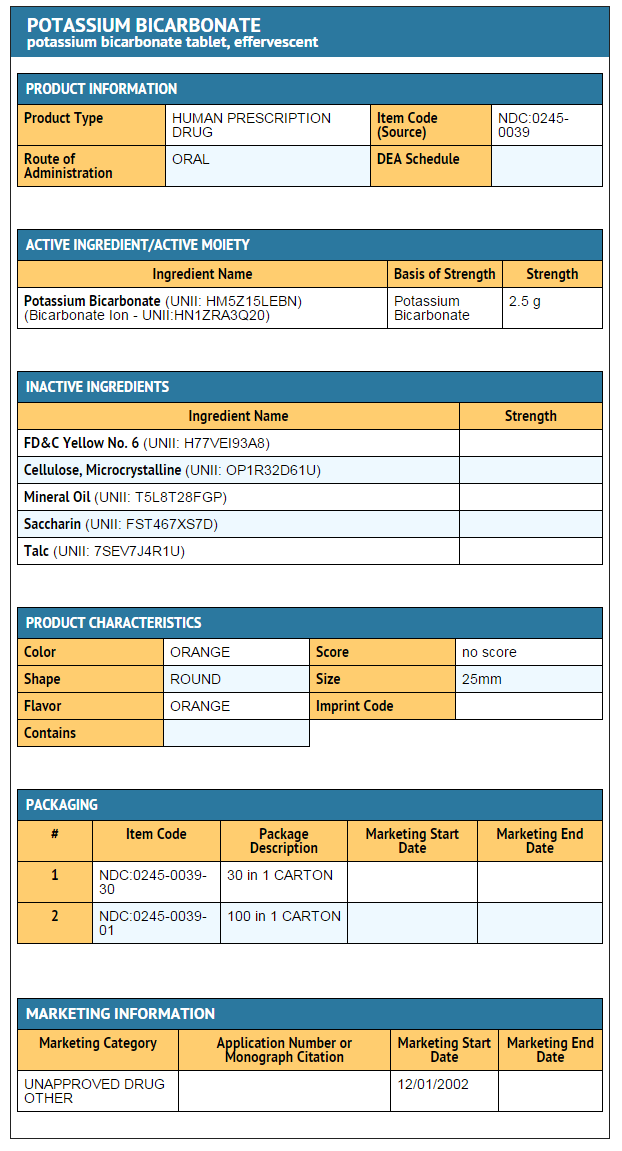
|structure=* Orange-flavored KLOR-CON®/EF Potassium Bicarbonate Effervescent Tablets for Oral Solution, USP are an oral potassium supplement offered as effervescent tablets in individual packets for dissolution in water. Each tablet contains potassium bicarbonate 2.5 g and citric acid 2 .1g which in solution provides 25 mEq (978 mg) potassium as bicarbonate and citrate. Also contains: FD&C Yellow No. 6, FD&C Yellow No. 6 Lake, microcrystalline cellulose, mineral oil, orange flavor, saccharin and talc. KLOR-CON®/EF tablets are sugar-free. | |||
There is limited information regarding <i> | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!-- | <!--Pharmacokinetics--> | ||
|PK=* The [[potassium]] ion is the principal intracellular cation of most body tissues. [[Potassium]] ions participate in a number of essential physiological processes, including the maintenance of [[intracellular]] [[tonicity]], the transmission of nerve impulses, the contraction of cardiac, skeletal and smooth muscle and the maintenance of normal renal function. | |||
* The [[intracellular]] concentration of potassium is approximately 150 to 160 mEq per liter. The normal adult plasma concentration is 3.5 to 5 mEq per liter. An active ion transport system maintains this gradient across the plasma membrane. | |||
* [[Potassium]] is a normal dietary constituent and under steady state conditions the amount of potassium absorbed from the [[gastrointestinal tract]] is equal to the amount excreted in the urine. The usual dietary intake of potassium is 50 to 100 mEq per day. | |||
* Potassium depletion will occur whenever the rate of potassium loss through renal excretion and/or loss from the gastrointestinal tract exceeds the rate of potassium intake. Such depletion usually develops as a consequence of therapy with diuretics, primary or secondary [[hyperaldosteronism]], [[diabetic ketoacidosis]] or inadequate replacement of potassium in patients on prolonged parenteral nutrition. Depletion can develop rapidly with severe diarrhea, especially if associated with [[vomiting]]. Potassium depletion due to these causes is usually accompanied by a concomitant loss of chloride and is manifested by hypokalemia and metabolic alkalosis. Potassium depletion may produce [[weakness]], [[fatigue]], disturbances of cardiac rhythm (primarily [[ectopic beats]]), prominent U-waves in the electrocardiogram and, in advanced cases, flaccid paralysis and/or impaired ability to concentrate urine. | |||
* If potassium depletion associated with [[metabolic alkalosis]] cannot be managed by correcting the fundamental cause of the deficiency (e.g., patients require long-term [[diuretic]] therapy), supplemental potassium in the form of high potassium food or potassium salts may be able to restore normal potassium levels. | |||
* In rare circumstances (e.g., patients with [[renal tubular acidosis]]), potassium depletion may be associated with [[metabolic acidosis]] and [[hyperchloremia]]. In such patients, potassium replacement should be accomplished with potassium salts such as potassium bicarbonate, potassium citrate, potassium acetate or potassium gluconate. | |||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic= | |nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | ||
* Carcinogenicity, mutagenicity and fertility studies in animals have not been performed. Potassium is a normal dietary constituent. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
| Line 313: | Line 211: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=* KLOR-CON®/EF Potassium Bicarbonate Effervescent Tablets for Oral Solution, USP are supplied in cartons of 30 individually wrapped tablets (NDC : 0245-0039-30) and cartons of 100 individually wrapped tablets (NDC 0245-0039-01). | ||
| | |storage=* Store at controlled room temperature, 15-30°C (59-86°F). | ||
| | |packLabel=====PRINCIPAL DISPLAY PANEL - 978 MG TABLET CARTON==== | ||
: [[File:Pot B PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!-- | ====Ingredients and Appearance==== | ||
: [[File:Pot B I n A.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=* Remind the patient to take this medicine following the frequency and amount prescribed. This is especially important if the patient is also taking diuretics and/or digitalis preparations. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* POTASSIUM BICARBONATE®<ref>{{Cite web | title = Potassium bicarbonate | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=89e3b51b-71a6-44ac-891e-5fa0e37e0a99}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |lookAlike=There is limited information regarding the look alike drug names. | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
Latest revision as of 16:58, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Potassium bicarbonate is a potassium supplement that is FDA approved for the treatment of hypokalemia, digitalis intoxication, hypokalemic familial periodic paralysis. Common adverse reactions include nausea, vomiting, flatulence, abdominal pain/discomfort and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- For the treatment of patients with hypokalemia, with or without metabolic alkalosis; in digitalis intoxication; and in patients with hypokalemic familial periodic paralysis. If hypokalemia is the result of diuretic therapy, consideration should be given to the use of a lower dose of diuretic, which may be sufficient without leading to hypokalemia.
- For the prevention of hypokalemia in patients who would be at particular risk if hypokalemia were to develop (e.g., digitalized patients or patients with significant cardiac arrhythmias).
- The use of potassium salts in patients receiving diuretics for uncomplicated essential hypertension is often unnecessary when such patients have a normal dietary pattern and when low doses of the diuretic are used. Serum potassium should be checked periodically, however, and if hypokalemia occurs, dietary supplementation with potassium-containing foods may be adequate to control milder cases. In more severe cases, and if dose adjustment of the diuretic is ineffective or unwarranted, supplementation with potassium salts may be indicated.
Dosage
- The usual dietary potassium intake by the average adult is 50 to 100 mEq per day. Potassium depletion sufficient to cause hypokalemia usually requires the loss of 200 mEq or more of potassium from the total body store.
- Dosage must be adjusted to the individual needs of each patient. The dose for the prevention of hypokalemia is typically 25 mEq per day. Doses of 50 to 100 mEq per day or more are used for the treatment of potassium depletion. Dosage should be divided if more than 25 mEq per day is given such that no more than 25 mEq is given in a single dose.
- The usual adult dose is 25 to 100 mEq of potassium per day (one KLOR-CON®/EF tablet 1 to 4 times daily after meals).
- Each KLOR-CON®/EF tablet should be dissolved in at least 4 ounces of cold or ice water. These preparations, like other potassium supplements, must be properly diluted to avoid the possibility of gastrointestinal irritation.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Potassium bicarbonate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Potassium bicarbonate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in children have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Potassium bicarbonate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Potassium bicarbonate in pediatric patients.
Contraindications
- Potassium supplements are contraindicated in patients with hyperkalemia since a further increase in serum potassium concentration in such patients can produce cardiac arrest. Hyperkalemia may complicate any of the following conditions: chronic renal failure, systemic acidosis such as diabetic acidosis, acute dehydration, extensive tissue breakdown as in severe burns, adrenal insufficiency or the administration of a potassium-sparing diuretic (e.g., spironolactone, triamterene or amiloride)
Warnings
Hyperkalemia
- In patients with impaired mechanisms for excreting potassium, the administration of potassium salts can produce hyperkalemia and cardiac arrest. This occurs most commonly in patients given potassium by the intravenous route but may also occur in patients given potassium orally. Potentially fatal hyperkalemia can develop rapidly and be asymptomatic. The use of potassium salts in patients with chronic renal disease, or any other condition which impairs potassium excretion, requires particularly careful monitoring of the serum potassium concentration and appropriate dosage adjustment.
Interaction with Potassium-Sparing Diuretics
- Hypokalemia should not be treated by the concomitant administration of potassium salts and a potassium-sparing diuretic (e.g., spironolactone, triamterene or amiloride), since the simultaneous administration of these agents can produce severe hyperkalemia.
Interaction with Angiotensin Converting Enzyme Inhibitors
- Angiotensin converting enzyme (ACE) inhibitors (e.g., captopril, enalapril) will produce some potassium retention by inhibiting aldosterone production. Potassium supplements should be given to patients receiving ACE inhibitors only with close monitoring.
Metabolic Acidosis
- Hypokalemia in patients with metabolic acidosis should be treated with an alkalinizing potassium salt such as potassium bicarbonate, potassium citrate, potassium acetate or potassium gluconate.
Precautions
General
- The diagnosis of potassium depletion is ordinarily made by demonstrating hypokalemia in a patient with a clinical history suggesting some cause for potassium depletion. In interpreting the serum potassium level, the physician should be aware that acute alkalosis per se can produce hypokalemia in the absence of a deficit in total body potassium while acute acidosis per se can increase the serum potassium concentration into the normal range even in the presence of a reduced total body potassium. The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease or acidosis, requires careful attention to acid-base balance and appropriate monitoring of serum electrolytes, the electrocardiogram and the clinical status of the patient.
Information for Patients
- Remind the patient to take this medicine following the frequency and amount prescribed. This is especially important if the patient is also taking diuretics and/or digitalis preparations.
Laboratory Tests
- When blood is drawn for analysis of plasma potassium, artifactual elevations can occur after improper venipuncture technique or as a result of in vitro hemolysis of the sample.
Adverse Reactions
Clinical Trials Experience
- One of the most severe adverse effects is hyperkalemia
- The most common adverse reactions to oral potassium salts are nausea, vomiting, flatulence, abdominal pain/discomfort and diarrhea. These symptoms are due to irritation of the gastrointestinal tract and are best managed by diluting the preparation further, taking the dose with meals or reducing the dose.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Potassium bicarbonate in the drug label.
Drug Interactions
- Potassium-sparing diuretics, angiotensin converting enzyme inhibitors
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with potassium salts. It is not known if potassium salts cause fetal harm when administered to a pregnant woman or affect reproductive capacity. Potassium supplements should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Potassium bicarbonate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Potassium bicarbonate during labor and delivery.
Nursing Mothers
- Many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from oral potassium supplements, a decision should be made whether to discontinue nursing or discontinue the drug, considering the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in children have not been established.
Geriatic Use
There is no FDA guidance on the use of Potassium bicarbonate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Potassium bicarbonate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Potassium bicarbonate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Potassium bicarbonate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Potassium bicarbonate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Potassium bicarbonate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Potassium bicarbonate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- The use of potassium salts in patients with chronic renal disease, or any other condition which impairs potassium excretion, requires particularly careful monitoring of the serum potassium concentration and appropriate dosage adjustment.
- Potassium supplements should be given to patients receiving ACE inhibitors only with close monitoring.
- The treatment of potassium depletion, particularly in the presence of cardiac disease, renal disease or acidosis, requires careful attention to acid-base balance and appropriate monitoring of serum electrolytes, the electrocardiogram and the clinical status of the patient.
IV Compatibility
There is limited information regarding IV Compatibility of Potassium bicarbonate in the drug label.
Overdosage
- The administration of oral potassium salts to persons with normal excretory mechanisms for potassium rarely causes serious hyperkalemia. However, if excretory mechanisms are impaired or if potassium is administered too rapidly intravenously, potentially fatal hyperkalemia can result. It is important to recognize that hyperkalemia is usually asymptomatic and may be manifested only by an increased serum potassium concentration (6.5-8.0 mEq/L) and characteristic electrocardiographic changes (peaking of T-waves, loss of P-wave, depression of S-T segment and prolongation of the QT interval). Late manifestations include muscle paralysis and cardiovascular collapse from cardiac arrest (9-12 mEq/L).
- Treatment measures for hyperkalemia include the following: 1) elimination of foods and medications containing potassium and elimination of potassium-sparing diuretics; 2) intravenous administration of 300 to 500 mL/hr of 10% dextrose solution containing 10 to 20 units of crystalline insulin per 1,000 mL; 3) correction of acidosis, if present, with intravenous sodium bicarbonate; 4) use of exchange resins, hemodialysis or peritoneal dialysis.
- In treating hyperkalemia in patients who have been stabilized on digitalis, too rapid a lowering of the serum potassium concentration can produce digitalis toxicity.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox ECNumberTemplate:Chembox E numberTemplate:Chembox AppearanceTemplate:Chembox OdourTemplate:Chembox DensityTemplate:Chembox MeltingPtTemplate:Chembox SolubilityInWaterTemplate:Chembox SolubilityTemplate:Chembox pKaTemplate:Chembox ThermochemistryTemplate:Chembox RPhrasesTemplate:Chembox NFPATemplate:Chembox FlashPtTemplate:Chembox Lethal amounts (set)Template:Chembox OtherAnionsTemplate:Chembox OtherCationsTemplate:Chembox Supplement| Template:Chembox header2 | Potassium bicarbonate | |
|---|---|
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| |
| |
| Properties | |
| KHCO3 | |
| Molar mass | 100.115 g/mol |
| Hazards | |
| Related compounds | |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
Structure
- Orange-flavored KLOR-CON®/EF Potassium Bicarbonate Effervescent Tablets for Oral Solution, USP are an oral potassium supplement offered as effervescent tablets in individual packets for dissolution in water. Each tablet contains potassium bicarbonate 2.5 g and citric acid 2 .1g which in solution provides 25 mEq (978 mg) potassium as bicarbonate and citrate. Also contains: FD&C Yellow No. 6, FD&C Yellow No. 6 Lake, microcrystalline cellulose, mineral oil, orange flavor, saccharin and talc. KLOR-CON®/EF tablets are sugar-free.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Potassium bicarbonate in the drug label.
Pharmacokinetics
- The potassium ion is the principal intracellular cation of most body tissues. Potassium ions participate in a number of essential physiological processes, including the maintenance of intracellular tonicity, the transmission of nerve impulses, the contraction of cardiac, skeletal and smooth muscle and the maintenance of normal renal function.
- The intracellular concentration of potassium is approximately 150 to 160 mEq per liter. The normal adult plasma concentration is 3.5 to 5 mEq per liter. An active ion transport system maintains this gradient across the plasma membrane.
- Potassium is a normal dietary constituent and under steady state conditions the amount of potassium absorbed from the gastrointestinal tract is equal to the amount excreted in the urine. The usual dietary intake of potassium is 50 to 100 mEq per day.
- Potassium depletion will occur whenever the rate of potassium loss through renal excretion and/or loss from the gastrointestinal tract exceeds the rate of potassium intake. Such depletion usually develops as a consequence of therapy with diuretics, primary or secondary hyperaldosteronism, diabetic ketoacidosis or inadequate replacement of potassium in patients on prolonged parenteral nutrition. Depletion can develop rapidly with severe diarrhea, especially if associated with vomiting. Potassium depletion due to these causes is usually accompanied by a concomitant loss of chloride and is manifested by hypokalemia and metabolic alkalosis. Potassium depletion may produce weakness, fatigue, disturbances of cardiac rhythm (primarily ectopic beats), prominent U-waves in the electrocardiogram and, in advanced cases, flaccid paralysis and/or impaired ability to concentrate urine.
- If potassium depletion associated with metabolic alkalosis cannot be managed by correcting the fundamental cause of the deficiency (e.g., patients require long-term diuretic therapy), supplemental potassium in the form of high potassium food or potassium salts may be able to restore normal potassium levels.
- In rare circumstances (e.g., patients with renal tubular acidosis), potassium depletion may be associated with metabolic acidosis and hyperchloremia. In such patients, potassium replacement should be accomplished with potassium salts such as potassium bicarbonate, potassium citrate, potassium acetate or potassium gluconate.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity, mutagenicity and fertility studies in animals have not been performed. Potassium is a normal dietary constituent.
Clinical Studies
There is limited information regarding Clinical Studies of Potassium bicarbonate in the drug label.
How Supplied
- KLOR-CON®/EF Potassium Bicarbonate Effervescent Tablets for Oral Solution, USP are supplied in cartons of 30 individually wrapped tablets (NDC : 0245-0039-30) and cartons of 100 individually wrapped tablets (NDC 0245-0039-01).
Storage
- Store at controlled room temperature, 15-30°C (59-86°F).
Images
Drug Images
{{#ask: Page Name::Potassium bicarbonate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 978 MG TABLET CARTON
Ingredients and Appearance
{{#ask: Label Page::Potassium bicarbonate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Remind the patient to take this medicine following the frequency and amount prescribed. This is especially important if the patient is also taking diuretics and/or digitalis preparations.
Precautions with Alcohol
- Alcohol-Potassium bicarbonate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- POTASSIUM BICARBONATE®[2]
Look-Alike Drug Names
There is limited information regarding the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Goldberg, Robert N.; Kishore, Nand; Lennen, Rebecca M. (2003). "Thermodynamic quantities for the ionization reactions of buffers in water". In David R. Lide. CRC handbook of chemistry and physics (84th ed.). Boca Raton, FL: CRC Press. pp. 7–13. ISBN 978-0-8493-0595-5. Retrieved 6 March 2011.
- ↑ "Potassium bicarbonate".