Pentazocine: Difference between revisions
No edit summary |
No edit summary |
||
| (9 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=Anesthesia; Adjunct: 30 mg IV, IM or SC every 3 to 4 hr as needed, | |fdaLIADAdult= | ||
Labor pain: a single 30 mg/dose IM (most common); | * Anesthesia; Adjunct: 30 mg IV, IM or SC every 3 to 4 hr as needed, max 360 mg/day; doses above 30 mg IV or 60 mg IM, SC are not recommended. | ||
Pain ( | * Labor pain: a single 30 mg/dose IM (most common); or 20 mg/dose IV for 2-3 doses at 2-3-hr intervals, as needed, after contractions have become regular. | ||
* Pain (moderate to severe): 30 mg IV, IM or SC every 3-4 hr as needed, max 360 mg/day; doses above 30 mg IV or 60 mg IM, SC are not recommended. | |||
|offLabelAdultGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Pentazocine in adult patients. | |offLabelAdultGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Pentazocine in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Pentazocine in adult patients. | |offLabelAdultNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Pentazocine in adult patients. | ||
|fdaLIADPed=Safety and effectiveness in children less than 12 yr of age not established | |fdaLIADPed=* Safety and effectiveness in children less than 12 yr of age not established. | ||
Anesthesia; | * Anesthesia; adjunct: single 0.5 mg/kg IM dose | ||
|offLabelPedGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Pentazocine in pediatric patients. | |offLabelPedGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Pentazocine in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Pentazocine in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Pentazocine in pediatric patients. | ||
|contraindications= | |||
* Pentazocine should not be administered to patients who are hypersensitive to it. | |||
|warnings= | |||
=====Drug Dependence===== | |||
* Special care should be exercised in prescribing pentazocine for emotionally unstable patients and for those with a history of drug misuse. Such patients should be closely supervised when greater than 4 or 5 days of therapy is contemplated. There have been instances of psychological and physical dependence on pentazocine in patients with such a history and, rarely, in patients without such a history. Extended use of parenteral pentazocine may lead to physical or psychological dependence in some patients. When pentazocine is abruptly discontinued, withdrawal symptoms such as abdominal cramps, elevated temperature, rhinorrhea, restlessness, anxiety, and lacrimation may occur. However, even when these have occurred, discontinuance has been accomplished with minimal difficulty. In the rare patient in whom more than minor difficulty has been encountered, reinstitution of parenteral pentazocine with gradual withdrawal has ameliorated the patient’s symptoms. Substituting methadone or other narcotics for pentazocine in the treatment of the pentazocine abstinence syndrome should be avoided. There have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy. | |||
* In prescribing parenteral pentazocine for chronic use, particularly if the drug is to be self-administered, the physician should take precautions to avoid increases in dose and frequency of injection by the patient. | |||
* Just as with all medication, the oral form of pentazocine is preferable for chronic administration. | |||
* Tissue Damage at Injection Sites: Severe sclerosis of the skin, subcutaneous tissues, and underlying muscle have occurred at the injection sites of patients who have received multiple doses of pentazocine lactate. Constant rotation of injection sites is, therefore, essential. In addition, animal studies have demonstrated that pentazocine is tolerated less well subcutaneously than intramuscularly. (See Dosage and Administration.) | |||
=====Head Injury and Increased Intracranial Pressure===== | |||
* As in the case of other potent analgesics, the potential of pentazocine injection for elevating [[cerebrospinal fluid]] pressure may be attributed to CO2 retention due to the respiratory depressant effects of the drug. These effects may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a preexisting increase in intracranial pressure. Furthermore, pentazocine can produce effects which may obscure the clinical course of patients with head injuries. In such patients, pentazocine must be used with extreme caution and only if its use is deemed essential. | |||
=====Acute CNS Manifestations===== | |||
* Patients receiving therapeutic doses of pentazocine have experienced [[hallucinations]] (usually [[visual]]), [[disorientation]], and [[confusion]] which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be closely observed and vital signs checked. If the drug is reinstituted, it should be done with caution since these acute CNS manifestations may recur. | |||
* Due to the potential for increased CNS depressant effects, alcohol should be used with caution in patients who are currently receiving pentazocine. | |||
=====Ambulatory Patients===== | |||
* Since [[sedation]], [[dizziness]], and occasional [[euphoria]] have been noted, ambulatory patients should be warned not to operate machinery, drive cars, or unnecessarily expose themselves to hazards. | |||
=====Myocardial Infarction===== | |||
* Caution should be exercised in the intravenous use of pentazocine for patients with [[acute myocardial infarction]] accompanied by [[hypertension]] or [[left ventricular failure]]. Data suggest that intravenous administration of pentazocine increases systemic and pulmonary arterial pressure and systemic vascular resistance in patients with acute myocardial infarction. | |||
=====NOTE===== | |||
* Acetone sodium bisulfite, a sulfite that may cause allergic-type reactions including [[anaphylactic symptoms]] and life-threatening or less severe asthmatic episodes in certain susceptible people, is contained in multiple-dose vials. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people. | |||
* The ampuls in the Uni-Amp™ Pak do not contain acetone sodium bisulfite. | |||
|clinicalTrials= | |||
* The most commonly occurring reactions are: [[nausea]], [[dizziness]] or [[lightheadedness]], [[vomiting]], [[euphoria]]. | |||
=====Dermatologic Reactions===== | |||
* Soft tissue [[induration]], [[nodules]], and cutaneous depression can occur at injection sites. [[Ulceration]] ([[sloughing]]) and severe sclerosis of the skin and subcutaneous tissues (and, rarely, underlying muscle) have been reported after multiple doses. Other reported dermatologic reactions include [[diaphoresis]], sting on injection, flushed skin including [[plethora]], [[dermatitis]] including [[pruritus]]. | |||
* Infrequently occurring reactions are—respiratory: [[respiratory depression]], [[dyspnea]], transient apnea in a small number of newborn infants whose mothers received pentazocine during labor; [[cardiovascular]]: [[circulatory depression]], [[shock]], [[hypertension]]; CNS effects: [[dizziness]], [[lightheadedness]], [[hallucinations]], [[sedation]], [[euphoria]], [[headache]], [[confusion]], [[disorientation]]; infrequently weakness, [[disturbed dreams]], [[insomnia]], [[syncope]], visual blurring and focusing difficulty, [[depression]]; and rarely [[tremor]], [[irritability]], [[excitement]], [[tinnitus]]; [[gastrointestinal]]: [[constipation]], [[dry mouth]]; other: [[urinary retention]], [[headache]], [[paresthesia]], alterations in rate or strength of uterine contractions during labor. | |||
* Rarely reported reactions include—[[neuromuscular]] and [[psychiatric]]: [[muscle tremor]], [[insomnia]], [[disorientation]], [[hallucinations]]; [[gastrointestinal]]: taste alteration, [[diarrhea]] and [[cramps]]; [[ophthalmic]]: [[blurred vision]], [[nystagmus]], [[diplopia]], [[miosis]]; [[hematologic]]: depression of [[white blood cells]] (especially [[granulocytes]]), which is usually reversible, moderate transient [[eosinophilia]]; other: tachycardia, weakness or faintness, chills; allergic reactions including edema of the face, [[toxic epidermal necrolysis]]. (See Acute CNS Manifestations and Drug Dependence under Warnings.) | |||
|useInPregnancyFDA= | |||
* Safe use of pentazocine during pregnancy (other than labor) has not been established. Animal reproduction studies have not demonstrated teratogenic or embryotoxic effects. However, pentazocine should be administered to pregnant patients (other than labor) only when, in the judgment of the physician, the potential benefits outweigh the possible hazards. Patients receiving pentazocine during labor have experienced no adverse effects other than those that occur with commonly used analgesics. pentazocine should be used with caution in women delivering premature infants. | |||
|useInPed= | |||
* The safety and efficacy of pentazocine as preoperative or preanesthetic medication have been established in pediatric patients 1 to 16 years of age. Use of pentazocine in these age groups is supported by evidence from adequate and controlled studies in adults with additional data from published controlled trials in pediatric patients. The safety and efficacy of pentazocine as a premedication for sedation have not been established in pediatric patients less than one year old. Information on the safety profile of pentazocine as a postoperative analgesic in children less than 16 years is limited. | |||
|useInGeri= | |||
* Elderly patients may be more sensitive to the analgesic effects of pentazocine than younger patients. (See Dosage and Administration.) | |||
* Clinical data indicate that differences in various pharmacokinetic parameters of pentazocine may exist between elderly and younger patients. (See Clinical Pharmacology.) | |||
* Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be started on low doses of pentazocine and observed closely. | |||
* This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | |||
|overdose= | |||
=====Manifestations===== | |||
* Clinical experience with pentazocine overdosage has been insufficient to define the signs of this condition. | |||
=====Treatment===== | |||
* Oxygen, [[intravenous fluids]], [[vasopressors]], and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered. For respiratory depression due to [[overdosage]] or unusual sensitivity to pentazocine, parenteral naloxone is a specific and effective antagonist. | |||
|drugBox={{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 464198314 | |||
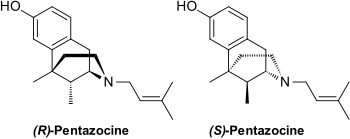
| IUPAC_name = <small>(2''RS'',6''RS'',11''RS'')-6,11-dimethyl-3-(3-methylbut-2-en-1-yl)-1,2,3,4,5,6-hexahydro-2,6-methano-3-benzazocin-8-ol</small><br />or<br /><small>2-dimethylallyl-5,9-dimethyl-2'-hydroxybenzomorphan</small> | |||
| image = Pentazocine wiki1.svg.png | |||
| width = 350px | |||
| imageL = Pentazocine image.gif | |||
| widthL = 175px | |||
| imageR = Pentazocine image.gif | |||
| widthR = 175px | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = RP4A60D26L | |||
| InChI = 1/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | |||
| InChIKey = VOKSWYLNZZRQPF-GDIGMMSIBX | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C19H27NO/c1-13(2)7-9-20-10-8-19(4)14(3)18(20)11-15-5-6-16(21)12-17(15)19/h5-7,12,14,18,21H,8-11H2,1-4H3/t14-,18+,19+/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = VOKSWYLNZZRQPF-GDIGMMSISA-N | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 359-83-1 | |||
| ATC_prefix = N02 | |||
| ATC_suffix = AD01 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 560 | |||
| PubChem = 441278 | |||
| IUPHAR_ligand = 1606 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00652 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 390041 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00498 | |||
| C = 19 |H = 27 |N = 1 |O = 1 | |||
| molecular_weight = 285.424 [[Gram|g]]/[[Mole (unit)|mol]] | |||
| smiles = Oc1ccc3c(c1)[C@]2([C@H]([C@H](N(CC2)C\C=C(/C)C)C3)C)C | |||
| bioavailability = ~20% orally | |||
| metabolism = [[Liver|Hepatic]] | |||
| elimination_half-life = 2 to 3 hours | |||
| excretion = [[Kidney|Renal]] | |||
| pregnancy_AU = C | |||
| pregnancy_US = C or D (if used near to term) | |||
| legal_AU = S8 | |||
| legal_US = Schedule IV | |||
| legal_status = Schedule III (international) | |||
| routes_of_administration = Oral, IV, IM | |||
}} | |||
|structure= | |||
* Pentazocine injection, Pentazocine Injection, USP, is a member of the benzazocine series (also known as the benzomorphan series). Chemically, pentazocine lactate is 1, 2, 3, 4, 5, 6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol lactate, a white, crystalline substance soluble in acidic aqueous solutions. | |||
|PD= | |||
* Pentazocine is a potent analgesic and 30 mg is usually as effective an analgesic as morphine 10 mg or meperidine 75 mg to 100 mg; however, a few studies suggest the Pentazocine to morphine ratio may range from 20 mg to 40 mg Pentazocine to 10 mg morphine. The duration of analgesia may sometimes be less than that of morphine. Analgesia usually occurs within 15 to 20 minutes after intramuscular or subcutaneous injection and within 2 to 3 minutes after intravenous injection. Pentazocine weakly antagonizes the analgesic effects of morphine, meperidine, and phenazocine; in addition, it produces incomplete reversal of cardiovascular, respiratory, and behavioral depression induced by morphine and meperidine. Pentazocine has about 1/50 the antagonistic activity of nalorphine. It also has sedative activity. | |||
|PK= | |||
* Clinical data indicate that differences in various pharmacokinetic parameters may be observed with increasing age. In one study, elderly patients exhibited a longer mean elimination half-life, a lower mean total plasma clearance, and a larger mean area under the concentration-time curve than younger patients. | |||
|howSupplied= | |||
[[File:Pentazocinehow supplied.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | |||
* The pH of Pentazocine solutions is adjusted between 4 and 5 with lactic acid or sodium hydroxide. The air in the ampuls and vials has been displaced by nitrogen gas. | |||
|storage= | |||
* Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] | |||
: Revised: September, 2010 | |||
: Printed in USA EN-2622 | |||
: Hospira, Inc., Lake Forest, IL 60045 USA | |||
|alcohol=Alcohol-Pentazocine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Pentazocine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | |||
{{LabelImage | |||
|fileName=Pentazocine label.png | |||
}} | }} | ||
Latest revision as of 02:10, 10 June 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pentazocine is an analgesic opioid that is FDA approved for the {{{indicationType}}} of anesthesia; adjunct, labor pain, pain (moderate to severe). Common adverse reactions include gastrointestinal: nausea, vomiting, neurologic: dizziness, lightheadedness, psychiatric: euphoria.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Anesthesia; Adjunct: 30 mg IV, IM or SC every 3 to 4 hr as needed, max 360 mg/day; doses above 30 mg IV or 60 mg IM, SC are not recommended.
- Labor pain: a single 30 mg/dose IM (most common); or 20 mg/dose IV for 2-3 doses at 2-3-hr intervals, as needed, after contractions have become regular.
- Pain (moderate to severe): 30 mg IV, IM or SC every 3-4 hr as needed, max 360 mg/day; doses above 30 mg IV or 60 mg IM, SC are not recommended.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Pentazocine in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Pentazocine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in children less than 12 yr of age not established.
- Anesthesia; adjunct: single 0.5 mg/kg IM dose
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Pentazocine in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Pentazocine in pediatric patients.
Contraindications
- Pentazocine should not be administered to patients who are hypersensitive to it.
Warnings
Drug Dependence
- Special care should be exercised in prescribing pentazocine for emotionally unstable patients and for those with a history of drug misuse. Such patients should be closely supervised when greater than 4 or 5 days of therapy is contemplated. There have been instances of psychological and physical dependence on pentazocine in patients with such a history and, rarely, in patients without such a history. Extended use of parenteral pentazocine may lead to physical or psychological dependence in some patients. When pentazocine is abruptly discontinued, withdrawal symptoms such as abdominal cramps, elevated temperature, rhinorrhea, restlessness, anxiety, and lacrimation may occur. However, even when these have occurred, discontinuance has been accomplished with minimal difficulty. In the rare patient in whom more than minor difficulty has been encountered, reinstitution of parenteral pentazocine with gradual withdrawal has ameliorated the patient’s symptoms. Substituting methadone or other narcotics for pentazocine in the treatment of the pentazocine abstinence syndrome should be avoided. There have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy.
- In prescribing parenteral pentazocine for chronic use, particularly if the drug is to be self-administered, the physician should take precautions to avoid increases in dose and frequency of injection by the patient.
- Just as with all medication, the oral form of pentazocine is preferable for chronic administration.
- Tissue Damage at Injection Sites: Severe sclerosis of the skin, subcutaneous tissues, and underlying muscle have occurred at the injection sites of patients who have received multiple doses of pentazocine lactate. Constant rotation of injection sites is, therefore, essential. In addition, animal studies have demonstrated that pentazocine is tolerated less well subcutaneously than intramuscularly. (See Dosage and Administration.)
Head Injury and Increased Intracranial Pressure
- As in the case of other potent analgesics, the potential of pentazocine injection for elevating cerebrospinal fluid pressure may be attributed to CO2 retention due to the respiratory depressant effects of the drug. These effects may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a preexisting increase in intracranial pressure. Furthermore, pentazocine can produce effects which may obscure the clinical course of patients with head injuries. In such patients, pentazocine must be used with extreme caution and only if its use is deemed essential.
Acute CNS Manifestations
- Patients receiving therapeutic doses of pentazocine have experienced hallucinations (usually visual), disorientation, and confusion which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be closely observed and vital signs checked. If the drug is reinstituted, it should be done with caution since these acute CNS manifestations may recur.
- Due to the potential for increased CNS depressant effects, alcohol should be used with caution in patients who are currently receiving pentazocine.
Ambulatory Patients
- Since sedation, dizziness, and occasional euphoria have been noted, ambulatory patients should be warned not to operate machinery, drive cars, or unnecessarily expose themselves to hazards.
Myocardial Infarction
- Caution should be exercised in the intravenous use of pentazocine for patients with acute myocardial infarction accompanied by hypertension or left ventricular failure. Data suggest that intravenous administration of pentazocine increases systemic and pulmonary arterial pressure and systemic vascular resistance in patients with acute myocardial infarction.
NOTE
- Acetone sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people, is contained in multiple-dose vials. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
- The ampuls in the Uni-Amp™ Pak do not contain acetone sodium bisulfite.
Adverse Reactions
Clinical Trials Experience
- The most commonly occurring reactions are: nausea, dizziness or lightheadedness, vomiting, euphoria.
Dermatologic Reactions
- Soft tissue induration, nodules, and cutaneous depression can occur at injection sites. Ulceration (sloughing) and severe sclerosis of the skin and subcutaneous tissues (and, rarely, underlying muscle) have been reported after multiple doses. Other reported dermatologic reactions include diaphoresis, sting on injection, flushed skin including plethora, dermatitis including pruritus.
- Infrequently occurring reactions are—respiratory: respiratory depression, dyspnea, transient apnea in a small number of newborn infants whose mothers received pentazocine during labor; cardiovascular: circulatory depression, shock, hypertension; CNS effects: dizziness, lightheadedness, hallucinations, sedation, euphoria, headache, confusion, disorientation; infrequently weakness, disturbed dreams, insomnia, syncope, visual blurring and focusing difficulty, depression; and rarely tremor, irritability, excitement, tinnitus; gastrointestinal: constipation, dry mouth; other: urinary retention, headache, paresthesia, alterations in rate or strength of uterine contractions during labor.
- Rarely reported reactions include—neuromuscular and psychiatric: muscle tremor, insomnia, disorientation, hallucinations; gastrointestinal: taste alteration, diarrhea and cramps; ophthalmic: blurred vision, nystagmus, diplopia, miosis; hematologic: depression of white blood cells (especially granulocytes), which is usually reversible, moderate transient eosinophilia; other: tachycardia, weakness or faintness, chills; allergic reactions including edema of the face, toxic epidermal necrolysis. (See Acute CNS Manifestations and Drug Dependence under Warnings.)
Postmarketing Experience
There is limited information regarding Pentazocine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Pentazocine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Safe use of pentazocine during pregnancy (other than labor) has not been established. Animal reproduction studies have not demonstrated teratogenic or embryotoxic effects. However, pentazocine should be administered to pregnant patients (other than labor) only when, in the judgment of the physician, the potential benefits outweigh the possible hazards. Patients receiving pentazocine during labor have experienced no adverse effects other than those that occur with commonly used analgesics. pentazocine should be used with caution in women delivering premature infants.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pentazocine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pentazocine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Pentazocine in women who are nursing.
Pediatric Use
- The safety and efficacy of pentazocine as preoperative or preanesthetic medication have been established in pediatric patients 1 to 16 years of age. Use of pentazocine in these age groups is supported by evidence from adequate and controlled studies in adults with additional data from published controlled trials in pediatric patients. The safety and efficacy of pentazocine as a premedication for sedation have not been established in pediatric patients less than one year old. Information on the safety profile of pentazocine as a postoperative analgesic in children less than 16 years is limited.
Geriatic Use
- Elderly patients may be more sensitive to the analgesic effects of pentazocine than younger patients. (See Dosage and Administration.)
- Clinical data indicate that differences in various pharmacokinetic parameters of pentazocine may exist between elderly and younger patients. (See Clinical Pharmacology.)
- Sedating drugs may cause confusion and oversedation in the elderly; elderly patients generally should be started on low doses of pentazocine and observed closely.
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Pentazocine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pentazocine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pentazocine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pentazocine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pentazocine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pentazocine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Pentazocine Administration in the drug label.
Monitoring
There is limited information regarding Pentazocine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pentazocine and IV administrations.
Overdosage
Manifestations
- Clinical experience with pentazocine overdosage has been insufficient to define the signs of this condition.
Treatment
- Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered. For respiratory depression due to overdosage or unusual sensitivity to pentazocine, parenteral naloxone is a specific and effective antagonist.
Pharmacology
Mechanism of Action
There is limited information regarding Pentazocine Mechanism of Action in the drug label.
Structure
- Pentazocine injection, Pentazocine Injection, USP, is a member of the benzazocine series (also known as the benzomorphan series). Chemically, pentazocine lactate is 1, 2, 3, 4, 5, 6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol lactate, a white, crystalline substance soluble in acidic aqueous solutions.
Pharmacodynamics
- Pentazocine is a potent analgesic and 30 mg is usually as effective an analgesic as morphine 10 mg or meperidine 75 mg to 100 mg; however, a few studies suggest the Pentazocine to morphine ratio may range from 20 mg to 40 mg Pentazocine to 10 mg morphine. The duration of analgesia may sometimes be less than that of morphine. Analgesia usually occurs within 15 to 20 minutes after intramuscular or subcutaneous injection and within 2 to 3 minutes after intravenous injection. Pentazocine weakly antagonizes the analgesic effects of morphine, meperidine, and phenazocine; in addition, it produces incomplete reversal of cardiovascular, respiratory, and behavioral depression induced by morphine and meperidine. Pentazocine has about 1/50 the antagonistic activity of nalorphine. It also has sedative activity.
Pharmacokinetics
- Clinical data indicate that differences in various pharmacokinetic parameters may be observed with increasing age. In one study, elderly patients exhibited a longer mean elimination half-life, a lower mean total plasma clearance, and a larger mean area under the concentration-time curve than younger patients.
Nonclinical Toxicology
There is limited information regarding Pentazocine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Pentazocine Clinical Studies in the drug label.
How Supplied

- The pH of Pentazocine solutions is adjusted between 4 and 5 with lactic acid or sodium hydroxide. The air in the ampuls and vials has been displaced by nitrogen gas.
Storage
- Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
- Revised: September, 2010
- Printed in USA EN-2622
- Hospira, Inc., Lake Forest, IL 60045 USA
Images
Drug Images
{{#ask: Page Name::Pentazocine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pentazocine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pentazocine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pentazocine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Pentazocine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Pentazocine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Pentazocine |Label Name=Pentazocine label.png
}}